Recombinant interleukin-1 receptor antagonist conjugated to superparamagnetic iron oxide nanoparticles for theranostic targeting of experimental glioblastoma
- PMID: 25622897
- PMCID: PMC4309733
- DOI: 10.1016/j.neo.2014.11.001
Recombinant interleukin-1 receptor antagonist conjugated to superparamagnetic iron oxide nanoparticles for theranostic targeting of experimental glioblastoma
Abstract
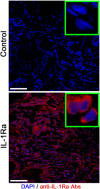
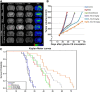
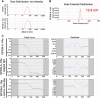
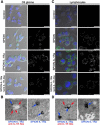
Cerebral edema commonly accompanies brain tumors and contributes to neurologic symptoms. The role of the interleukin-1 receptor antagonist conjugated to superparamagnetic iron oxide nanoparticles (SPION-IL-1Ra) was assessed to analyze its anti-edemal effect and its possible application as a negative contrast enhancing agent for magnetic resonance imaging (MRI). Rats with intracranial C6 glioma were intravenously administered at various concentrations of IL-1Ra or SPION-IL-1Ra. Brain peritumoral edema following treatment with receptor antagonist was assessed with high-field MRI. IL-1Ra administered at later stages of tumor progression significantly reduced peritumoral edema (as measured by MRI) and prolonged two-fold the life span of comorbid animals in a dose-dependent manner in comparison to control and corticosteroid-treated animals (P < .001). Synthesized SPION-IL-1Ra conjugates had the properties of negative contrast agent with high coefficients of relaxation efficiency. In vitro studies of SPION-IL-1Ra nanoparticles demonstrated high intracellular incorporation and absence of toxic influence on C6 cells and lymphocyte viability and proliferation. Retention of the nanoparticles in the tumor resulted in enhanced hypotensive T2-weighted images of glioma, proving the application of the conjugates as negative magnetic resonance contrast agents. Moreover, nanoparticles reduced the peritumoral edema confirming the therapeutic potency of synthesized conjugates. SPION-IL-1Ra nanoparticles have an anti-edemal effect when administered through a clinically relevant route in animals with glioma. The SPION-IL-1Ra could be a candidate for theranostic approach in neuro-oncology both for diagnosis of brain tumors and management of peritumoral edema.
Copyright © 2014 Neoplasia Press, Inc. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Schoenegger K., Oberndorfer S., Wuschitz B., Struhal W., Hainfellner J., Prayer D., Heinzl H., Lahrmann H., Marosi C., Grisold W. Peritumoral edema on MRI at initial diagnosis: an independent prognostic factor for glioblastoma? Eur J Neurol. 2009;16:874–878. - PubMed
-
- Koehler P.J. Use of corticosteroids in neuro-oncology. Anticancer Drugs. 1995;16:19–33. - PubMed
-
- Roth P., Regli L., Tonder M., Weller M. Tumor-associated edema in brain cancer patients: pathogenesis and management. Expert Rev Anticancer Ther. 2013;13:1319–1325. - PubMed
-
- Badie B., Schartner J.M., Hagar A.R., Prabakaran S., Peebles T.R., Bartley B., Lapsiwala S., Resnick D.K., Vorpahl J. Microglia cyclooxygenase-2 activity in experimental gliomas: possible role in cerebral edema formation. Clin Cancer Res. 2003;9:872–877. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

