Suppression of tumor growth in mice by rationally designed pseudopeptide inhibitors of fibroblast activation protein and prolyl oligopeptidase
- PMID: 25622898
- PMCID: PMC4309729
- DOI: 10.1016/j.neo.2014.11.002
Suppression of tumor growth in mice by rationally designed pseudopeptide inhibitors of fibroblast activation protein and prolyl oligopeptidase
Abstract
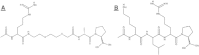
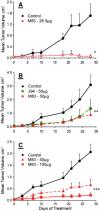
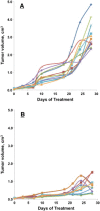
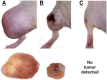
Tumor microenvironments (TMEs) are composed of cancer cells, fibroblasts, extracellular matrix, microvessels, and endothelial cells. Two prolyl endopeptidases, fibroblast activation protein (FAP) and prolyl oligopeptidase (POP), are commonly overexpressed by epithelial-derived malignancies, with the specificity of FAP expression by cancer stromal fibroblasts suggesting FAP as a possible therapeutic target. Despite overexpression in most cancers and having a role in angiogenesis, inhibition of POP activity has received little attention as an approach to quench tumor growth. We developed two specific and highly effective pseudopeptide inhibitors, M83, which inhibits FAP and POP proteinase activities, and J94, which inhibits only POP. Both suppressed human colon cancer xenograft growth >90% in mice. By immunohistochemical stains, M83- and J94-treated tumors had fewer microvessels, and apoptotic areas were apparent in both. In response to M83, but not J94, disordered collagen accumulations were observed. Neither M83- nor J94-treated mice manifested changes in behavior, weight, or gastrointestinal function. Tumor growth suppression was more extensive than noted with recently reported efforts by others to inhibit FAP proteinase function or reduce FAP expression. Diminished angiogenesis and the accompanying profound reduction in tumor growth suggest that inhibition of either FAP or POP may offer new therapeutic approaches that directly target TMEs.
Copyright © 2014 Neoplasia Press, Inc. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Targeting inhibition of fibroblast activation protein-α and prolyl oligopeptidase activities on cells common to metastatic tumor microenvironments.Neoplasia. 2013 Apr;15(4):348-58. doi: 10.1593/neo.121850. Neoplasia. 2013. PMID: 23555181 Free PMC article.
-
Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin.Mol Cancer Ther. 2009 May;8(5):1378-86. doi: 10.1158/1535-7163.MCT-08-1170. Epub 2009 May 5. Mol Cancer Ther. 2009. PMID: 19417147 Free PMC article.
-
Inhibitor-Decorated Polymer Conjugates Targeting Fibroblast Activation Protein.J Med Chem. 2017 Oct 26;60(20):8385-8393. doi: 10.1021/acs.jmedchem.7b00767. Epub 2017 Oct 16. J Med Chem. 2017. PMID: 28953383
-
Fibroblast activation protein-α in fibrogenic disorders and cancer: more than a prolyl-specific peptidase?Expert Opin Ther Targets. 2017 Oct;21(10):977-991. doi: 10.1080/14728222.2017.1370455. Epub 2017 Aug 30. Expert Opin Ther Targets. 2017. PMID: 28829211 Review.
-
Fibroblast activation protein-α: a key modulator of the microenvironment in multiple pathologies.Int Rev Cell Mol Biol. 2012;297:83-116. doi: 10.1016/B978-0-12-394308-8.00003-0. Int Rev Cell Mol Biol. 2012. PMID: 22608558 Review.
Cited by
-
Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics.Oncogene. 2018 Aug;37(32):4343-4357. doi: 10.1038/s41388-018-0275-3. Epub 2018 May 3. Oncogene. 2018. PMID: 29720723 Free PMC article. Review.
-
Prolyl endopeptidase inhibitor Y-29794 blocks the IRS1-AKT-mTORC1 pathway and inhibits survival and in vivo tumor growth of triple-negative breast cancer.Cancer Biol Ther. 2020 Nov 1;21(11):1033-1040. doi: 10.1080/15384047.2020.1824989. Epub 2020 Oct 12. Cancer Biol Ther. 2020. PMID: 33044914 Free PMC article.
-
Improved FAPI-radiopharmaceutical pharmacokinetics from the perspectives of a dose escalation study.Eur J Nucl Med Mol Imaging. 2025 Jul;52(9):3238-3251. doi: 10.1007/s00259-025-07141-1. Epub 2025 Feb 26. Eur J Nucl Med Mol Imaging. 2025. PMID: 40000459 Free PMC article.
-
Inhibitor-conjugated harmonic nanoparticles targeting fibroblast activation protein.RSC Adv. 2019 Oct 4;9(54):31659-31669. doi: 10.1039/c9ra05299b. eCollection 2019 Oct 1. RSC Adv. 2019. PMID: 35527932 Free PMC article.
-
Discovery of a Tunable Heterocyclic Electrophile 4-Chloro-pyrazolopyridine That Defines a Unique Subset of Ligandable Cysteines.ACS Chem Biol. 2024 May 17;19(5):1082-1092. doi: 10.1021/acschembio.4c00025. Epub 2024 Apr 17. ACS Chem Biol. 2024. PMID: 38629450 Free PMC article.
References
-
- Connolly J, Schnitt S, Wang H, Dvorak A, Dvorak H. Principles of Cancer Pathology. In: Bast RJ, Kufe D, Pollock R, Welchselbaum R, Holland J, Frei E, editors. Holland-Frei Cancer Medicine. 2000.
-
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. - PubMed
-
- Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274:36505–36512. - PubMed
-
- Cheng JD, Dunbrack RL, Jr., Valianou M, Rogatko A, Alpaugh RK, Weiner LM. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res. 2002;62:4767–4772. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

