Prediction of brain age suggests accelerated atrophy after traumatic brain injury
- PMID: 25623048
- PMCID: PMC4403966
- DOI: 10.1002/ana.24367
Prediction of brain age suggests accelerated atrophy after traumatic brain injury
Abstract
Objective: The long-term effects of traumatic brain injury (TBI) can resemble observed in normal ageing, suggesting that TBI may accelerate the ageing process. We investigate this using a neuroimaging model that predicts brain age in healthy individuals and then apply it to TBI patients. We define individuals' differences in chronological and predicted structural "brain age," and test whether TBI produces progressive atrophy and how this relates to cognitive function.
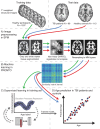
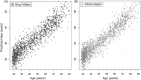
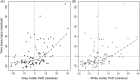
Methods: A predictive model of normal ageing was defined using machine learning in 1,537 healthy individuals, based on magnetic resonance imaging-derived estimates of gray matter (GM) and white matter (WM). This ageing model was then applied to test 99 TBI patients and 113 healthy controls to estimate brain age.
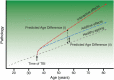
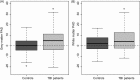
Results: The initial model accurately predicted age in healthy individuals (r = 0.92). TBI brains were estimated to be "older," with a mean predicted age difference (PAD) between chronological and estimated brain age of 4.66 years (±10.8) for GM and 5.97 years (±11.22) for WM. This PAD predicted cognitive impairment and correlated strongly with the time since TBI, indicating that brain tissue loss increases throughout the chronic postinjury phase.
Interpretation: TBI patients' brains were estimated to be older than their chronological age. This discrepancy increases with time since injury, suggesting that TBI accelerates the rate of brain atrophy. This may be an important factor in the increased susceptibility in TBI patients for dementia and other age-associated conditions, motivating further research into the age-like effects of brain injury and other neurological diseases.
© 2015 The Authors Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures

References
-
- Smith DH, Chen XH, Pierce JES. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma. 1997;14:715–727. - PubMed
-
- Moretti L, Cristofori I, Weaver SM. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012;11:1103–1112. - PubMed
-
- Bazarian JJ, Cernak I, Noble-Haeusslein L. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24:439–451. - PubMed
-
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

