Role of Δ1-pyrroline-5-carboxylate dehydrogenase supports mitochondrial metabolism and host-cell invasion of Trypanosoma cruzi
- PMID: 25623067
- PMCID: PMC4367278
- DOI: 10.1074/jbc.M114.574525
Role of Δ1-pyrroline-5-carboxylate dehydrogenase supports mitochondrial metabolism and host-cell invasion of Trypanosoma cruzi
Abstract
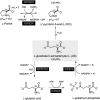
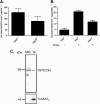
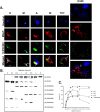
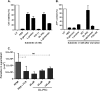
Proline is crucial for energizing critical events throughout the life cycle of Trypanosoma cruzi, the etiological agent of Chagas disease. The proline breakdown pathway consists of two oxidation steps, both of which produce reducing equivalents as follows: the conversion of proline to Δ(1)-pyrroline-5-carboxylate (P5C), and the subsequent conversion of P5C to glutamate. We have identified and characterized the Δ(1)-pyrroline-5-carboxylate dehydrogenase from T. cruzi (TcP5CDH) and report here on how this enzyme contributes to a central metabolic pathway in this parasite. Size-exclusion chromatography, two-dimensional gel electrophoresis, and small angle x-ray scattering analysis of TcP5CDH revealed an oligomeric state composed of two subunits of six protomers. TcP5CDH was found to complement a yeast strain deficient in PUT2 activity, confirming the enzyme's functional role; and the biochemical parameters (Km, kcat, and kcat/Km) of the recombinant TcP5CDH were determined, exhibiting values comparable with those from T. cruzi lysates. In addition, TcP5CDH exhibited mitochondrial staining during the main stages of the T. cruzi life cycle. mRNA and enzymatic activity levels indicated the up-regulation (6-fold change) of TcP5CDH during the infective stages of the parasite. The participation of P5C as an energy source was also demonstrated. Overall, we propose that this enzymatic step is crucial for the viability of both replicative and infective forms of T. cruzi.
Keywords: Bioenergetics; Enzyme Mechanism; Host-Pathogen Interaction; Mitochondrial Metabolism; Parasite Metabolism; Trypanosoma cruzi.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Trypanosoma cruzi synthesizes proline via a Δ1-pyrroline-5-carboxylate reductase whose activity is fine-tuned by NADPH cytosolic pools.Biochem J. 2020 May 29;477(10):1827-1845. doi: 10.1042/BCJ20200232. Biochem J. 2020. PMID: 32315030
-
Higher expression of proline dehydrogenase altered mitochondrial function and increased Trypanosoma cruzi differentiation in vitro and in the insect vector.Biochem J. 2021 Nov 12;478(21):3891-3903. doi: 10.1042/BCJ20210428. Biochem J. 2021. PMID: 34661234
-
Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes.J Biol Chem. 2009 Sep 25;284(39):26482-92. doi: 10.1074/jbc.M109.009340. Epub 2009 Jul 27. J Biol Chem. 2009. PMID: 19635803 Free PMC article.
-
P5C as an Interface of Proline Interconvertible Amino Acids and Its Role in Regulation of Cell Survival and Apoptosis.Int J Mol Sci. 2021 Oct 29;22(21):11763. doi: 10.3390/ijms222111763. Int J Mol Sci. 2021. PMID: 34769188 Free PMC article. Review.
-
Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens.Front Plant Sci. 2015 Jul 6;6:503. doi: 10.3389/fpls.2015.00503. eCollection 2015. Front Plant Sci. 2015. PMID: 26217357 Free PMC article. Review.
Cited by
-
Branched-chain amino acids modulate the proteomic profile of Trypanosoma cruzi metacyclogenesis induced by proline.PLoS Negl Trop Dis. 2024 Oct 9;18(10):e0012588. doi: 10.1371/journal.pntd.0012588. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39383181 Free PMC article.
-
Metabolomic profiling reveals a finely tuned, starvation-induced metabolic switch in Trypanosoma cruzi epimastigotes.J Biol Chem. 2017 May 26;292(21):8964-8977. doi: 10.1074/jbc.M117.778522. Epub 2017 Mar 29. J Biol Chem. 2017. PMID: 28356355 Free PMC article.
-
The Uptake and Metabolism of Amino Acids, and Their Unique Role in the Biology of Pathogenic Trypanosomatids.Pathogens. 2018 Apr 1;7(2):36. doi: 10.3390/pathogens7020036. Pathogens. 2018. PMID: 29614775 Free PMC article. Review.
-
The glutamine synthetase of Trypanosoma cruzi is required for its resistance to ammonium accumulation and evasion of the parasitophorous vacuole during host-cell infection.PLoS Negl Trop Dis. 2018 Jan 10;12(1):e0006170. doi: 10.1371/journal.pntd.0006170. eCollection 2018 Jan. PLoS Negl Trop Dis. 2018. PMID: 29320490 Free PMC article.
-
Diminished Prolinemia in Chronic Chagasic Patients: A New Clue for Disease Pathology?Molecules. 2019 Aug 30;24(17):3167. doi: 10.3390/molecules24173167. Molecules. 2019. PMID: 31480397 Free PMC article.
References
-
- Chagas C. (1909) Nova tripanozomiaze humana. Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz. 1, 159–218
-
- Brener Z. (1973) Biology of Trypanosoma cruzi. Annu. Rev. Microbiol. 27, 347–382 - PubMed
-
- World Health Organization (2012) in Epidemiology and Burden of Disease (www.who.int/iris/bitstream/10665/77472/1/WHO_TRS_975_eng.pdf)
-
- Brener Z. (1971) Life cycle of Trypanosoma cruzi. Rev. Inst. Med. Trop Sao. Paulo. 13, 171–178 - PubMed
-
- Bastos C. J., Aras R., Mota G., Reis F., Dias J. P., de Jesus R. S., Freire M. S., de Araújo E. G., Prazeres J., Grassi M. F. (2010) Clinical outcomes of thirteen patients with acute Chagas disease acquired through oral transmission from two urban outbreaks in northeastern Brazil. PLoS Negl. Trop. Dis. 4, e711. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

