Clinical and cost effectiveness of computer treatment for aphasia post stroke (Big CACTUS): study protocol for a randomised controlled trial
- PMID: 25623162
- PMCID: PMC4318176
- DOI: 10.1186/s13063-014-0527-7
Clinical and cost effectiveness of computer treatment for aphasia post stroke (Big CACTUS): study protocol for a randomised controlled trial
Abstract
Background: Aphasia affects the ability to speak, comprehend spoken language, read and write. One third of stroke survivors experience aphasia. Evidence suggests that aphasia can continue to improve after the first few months with intensive speech and language therapy, which is frequently beyond what resources allow. The development of computer software for language practice provides an opportunity for self-managed therapy. This pragmatic randomised controlled trial will investigate the clinical and cost effectiveness of a computerised approach to long-term aphasia therapy post stroke.
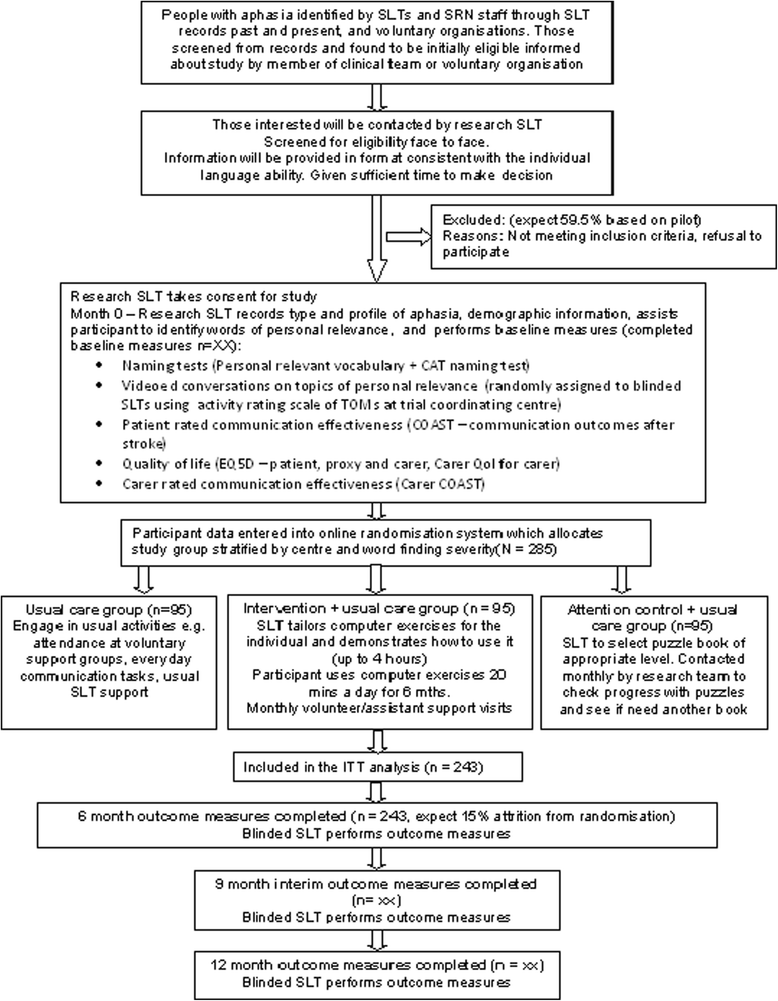
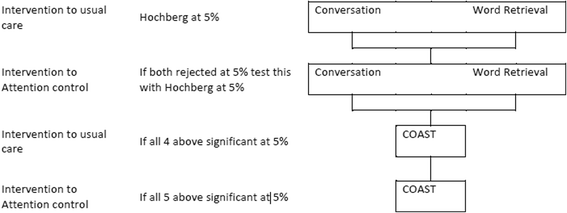
Methods/design: A total of 285 adults with aphasia at least four months post stroke will be randomly allocated to either usual care, computerised intervention in addition to usual care or attention and activity control in addition to usual care. Those in the intervention group will receive six months of self-managed word finding practice on their home computer with monthly face-to-face support from a volunteer/assistant. Those in the attention control group will receive puzzle activities, supplemented by monthly telephone calls. Study delivery will be coordinated by 20 speech and language therapy departments across the United Kingdom. Outcome measures will be made at baseline, six, nine and 12 months after randomisation by blinded speech and language therapist assessors. Primary outcomes are the change in number of words (of personal relevance) named correctly at six months and improvement in functional conversation. Primary outcomes will be analysed using a Hochberg testing procedure. Significance will be declared if differences in both word retrieval and functional conversation at six months are significant at the 5% level, or if either comparison is significant at 2.5%. A cost utility analysis will be undertaken from the NHS and personal social service perspective. Differences between costs and quality-adjusted life years in the three groups will be described and the incremental cost effectiveness ratio will be calculated. Treatment fidelity will be monitored.
Discussion: This is the first fully powered trial of the clinical and cost effectiveness of computerised aphasia therapy. Specific challenges in designing the protocol are considered.
Trial registration: Registered with Current Controlled Trials ISRCTN68798818 on 18 February 2014.
Figures
References
-
- Department of Health . National Stroke Strategy. London: Department of Health; 2007.
-
- Brady MC, Kelly H, Godwin J, Enderby P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. 2012;5 - PubMed
-
- Bowen A, Hesketh A, Patchick E, Young A, Davies L, Vail A, Long A, Watkins C, Wilkinson M, Pearl G, Lambon Ralph M, Tyrrell P. Clinical effectiveness, cost effectiveness and service users’ perceptions of early, well-resourced communication therapy following a stroke, a randomised controlled trial (The ACT NoW Study) Health Technol Assess. 2012;16:26. doi: 10.3310/hta16260. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical