An ex vivo approach to botanical-drug interactions: a proof of concept study
- PMID: 25623616
- PMCID: PMC4355093
- DOI: 10.1016/j.jep.2015.01.021
An ex vivo approach to botanical-drug interactions: a proof of concept study
Abstract
Ethnopharmacological relevance: Botanical medicines are frequently used in combination with therapeutic drugs, imposing a risk for harmful botanical-drug interactions (BDIs). Among the existing BDI evaluation methods, clinical studies are the most desirable, but due to their expense and protracted time-line for completion, conventional in vitro methodologies remain the most frequently used BDI assessment tools. However, many predictions generated from in vitro studies are inconsistent with clinical findings. Accordingly, the present study aimed to develop a novel ex vivo approach for BDI assessment and expand the safety evaluation methodology in applied ethnopharmacological research.
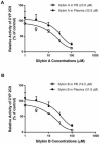
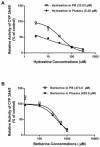
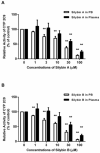
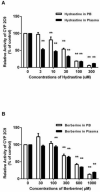
Materials and methods: This approach differs from conventional in vitro methods in that rather than botanical extracts or individual phytochemicals being prepared in artificial buffers, human plasma/serum collected from a limited number of subjects administered botanical supplements was utilized to assess BDIs. To validate the methodology, human plasma/serum samples collected from healthy subjects administered either milk thistle or goldenseal extracts were utilized in incubation studies to determine their potential inhibitory effects on CYP2C9 and CYP3A4/5, respectively. Silybin A and B, two principal milk thistle phytochemicals, and hydrastine and berberine, the purported active constituents in goldenseal, were evaluated in both phosphate buffer and human plasma based in vitro incubation systems.
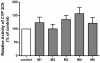
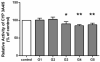
Results: Ex vivo study results were consistent with formal clinical study findings for the effect of milk thistle on the disposition of tolbutamide, a CYP2C9 substrate, and for goldenseal׳s influence on the pharmacokinetics of midazolam, a widely accepted CYP3A4/5 substrate. Compared to conventional in vitro BDI methodologies of assessment, the introduction of human plasma into the in vitro study model changed the observed inhibitory effect of silybin A, silybin B and hydrastine and berberine on CYP2C9 and CYP3A4/5, respectively, results which more closely mirrored those generated in clinical study.
Conclusions: Data from conventional buffer-based in vitro studies were less predictive than the ex vivo assessments. Thus, this novel ex vivo approach may be more effective at predicting clinically relevant BDIs than conventional in vitro methods.
Keywords: Botanical–drug interactions; Cytochrome P450s; Ex-vivo model; Goldenseal; Milk thistle.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures
Similar articles
-
The effects of milk thistle (Silybum marianum) on human cytochrome P450 activity.Drug Metab Dispos. 2014 Oct;42(10):1611-6. doi: 10.1124/dmd.114.057232. Epub 2014 Jul 15. Drug Metab Dispos. 2014. PMID: 25028567 Free PMC article. Clinical Trial.
-
An in vitro evaluation of cytochrome P450 inhibition and P-glycoprotein interaction with goldenseal, Ginkgo biloba, grape seed, milk thistle, and ginseng extracts and their constituents.Planta Med. 2007 Jul;73(8):731-41. doi: 10.1055/s-2007-981550. Epub 2007 Jul 5. Planta Med. 2007. PMID: 17611934
-
The in-vitro effect of complementary and alternative medicines on cytochrome P450 2C9 activity.J Pharm Pharmacol. 2014 Sep;66(9):1339-46. doi: 10.1111/jphp.12259. Epub 2014 Apr 15. J Pharm Pharmacol. 2014. PMID: 24730468
-
Relevance of in vitro and clinical data for predicting CYP3A4-mediated herb-drug interactions in cancer patients.Cancer Treat Rev. 2013 Nov;39(7):773-83. doi: 10.1016/j.ctrv.2012.12.008. Epub 2013 Feb 8. Cancer Treat Rev. 2013. PMID: 23394826 Review.
-
Goldenseal (Hydrastis canadensis L.) and its active constituents: A critical review of their efficacy and toxicological issues.Pharmacol Res. 2020 Oct;160:105085. doi: 10.1016/j.phrs.2020.105085. Epub 2020 Jul 16. Pharmacol Res. 2020. PMID: 32683037 Review.
Cited by
-
Physiologically based pharmacokinetic model predictions of natural product-drug interactions between goldenseal, berberine, imatinib and bosutinib.Eur J Clin Pharmacol. 2022 Apr;78(4):597-611. doi: 10.1007/s00228-021-03266-y. Epub 2022 Jan 20. Eur J Clin Pharmacol. 2022. PMID: 35048143
-
Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance.Life (Basel). 2020 Jul 4;10(7):106. doi: 10.3390/life10070106. Life (Basel). 2020. PMID: 32635538 Free PMC article. Review.
-
Effects of Berberis vulgaris, and its active constituent berberine on cytochrome P450: a review.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jan;398(1):179-202. doi: 10.1007/s00210-024-03326-x. Epub 2024 Aug 14. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39141022 Review.
-
Herb-drug interactions: a novel algorithm-assisted information system for pharmacokinetic drug interactions with herbal supplements in cancer treatment.Eur J Clin Pharmacol. 2019 Sep;75(9):1237-1248. doi: 10.1007/s00228-019-02700-6. Epub 2019 Jun 1. Eur J Clin Pharmacol. 2019. PMID: 31154477
-
The Role of Natural Products in Diabetic Retinopathy.Biomedicines. 2024 May 21;12(6):1138. doi: 10.3390/biomedicines12061138. Biomedicines. 2024. PMID: 38927345 Free PMC article. Review.
References
-
- Abourashed EA, Khan IA. High-performance liquid chromatography determination of hydrastine and berberine in dietary supplements containing goldenseal. Journal of pharmaceutical sciences. 2001;90:817–822. - PubMed
-
- Agarwal A, D'Souza P, Johnson TS, Dethe SM, Chandrasekaran C. Use of in vitro bioassays for assessing botanicals. Current opinion in biotechnology. 2014;25:39–44. - PubMed
-
- Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Molecular & cellular proteomics : MCP. 2002;1:845–867. - PubMed
-
- Beckmann-Knopp S, Rietbrock S, Weyhenmeyer R, Bocker RH, Beckurts KT, Lang W, Hunz M, Fuhr U. Inhibitory effects of silibinin on cytochrome P-450 enzymes in human liver microsomes. Pharmacology & toxicology. 2000;86:250–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

