TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing
- PMID: 25623783
- PMCID: PMC4428840
- DOI: 10.1007/s00424-015-1689-1
TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing
Abstract
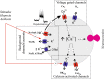
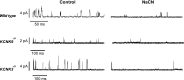
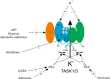
Arterial chemoreceptors play a vital role in cardiorespiratory control by providing the brain with information regarding blood oxygen, carbon dioxide, and pH. The main chemoreceptor, the carotid body, is composed of sensory (type 1) cells which respond to hypoxia or acidosis with a depolarising receptor potential which in turn activates voltage-gated calcium entry, neurosecretion and excitation of adjacent afferent nerves. The receptor potential is generated by inhibition of Twik-related acid-sensitive K(+) channel 1 and 3 (TASK1/TASK3) heterodimeric channels which normally maintain the cells' resting membrane potential. These channels are thought to be directly inhibited by acidosis. Oxygen sensitivity, however, probably derives from a metabolic signalling pathway. The carotid body, isolated type 1 cells, and all forms of TASK channel found in the type 1 cell, are highly sensitive to inhibitors of mitochondrial metabolism. Moreover, type1 cell TASK channels are activated by millimolar levels of MgATP. In addition to their role in the transduction of chemostimuli, type 1 cell TASK channels have also been implicated in the modulation of chemoreceptor function by a number of neurocrine/paracrine signalling molecules including adenosine, GABA, and serotonin. They may also be instrumental in mediating the depression of the acute hypoxic ventilatory response that occurs with some general anaesthetics. Modulation of TASK channel activity is therefore a key mechanism by which the excitability of chemoreceptors can be controlled. This is not only of physiological importance but may also offer a therapeutic strategy for the treatment of cardiorespiratory disorders that are associated with chemoreceptor dysfunction.
Figures
References
-
- Andres-Enguix I, Caley A, Yustos R, Schumacher MA, Spanu PD, Dickinson R, Maze M, Franks NP. Determinants of the anesthetic sensitivity of two-pore domain acid-sensitive potassium channels: molecular cloning of an anesthetic-activated potassium channel from Lymnaea stagnalis. J Biol Chem. 2007;282:20977–20990. - PubMed
-
- Anichkov S, Belen'kii M. Pharmacology of the carotid body chemoreceptors. Oxford: Pergamon; 1963.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

