The effect of injection using narrow-bore needles on mammalian cells: administration and formulation considerations for cell therapies
- PMID: 25623928
- PMCID: PMC4964945
- DOI: 10.1111/jphp.12362
The effect of injection using narrow-bore needles on mammalian cells: administration and formulation considerations for cell therapies
Abstract
Objectives: This study focuses on the effect of the injection administration process on a range of cell characteristics.
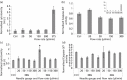
Methods: Effects of different ejection rates, needle sizes and cell suspension densities were assessed in terms of viability, membrane integrity, apoptosis and senescence of NIH 3T3 fibroblasts. For ratiometric measurements, a multiplex assay was used to verify cell viability, cytotoxicity and apoptosis independent of cell number. Co-delivery with alginate hydrogels and viscosity-modifying excipients was also assessed.
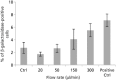
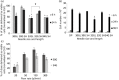
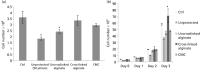
Key findings: Ejections at 150 μl/min resulted in the highest percentage of dose being delivered as viable cells among ejection rates tested. The difference in proportions of apoptotic cells became apparent 48 h after ejection, with proportions being higher in samples ejected at slower rates. Co-delivery with alginate hydrogels demonstrated a protective action on the cell payload.
Conclusions: This study demonstrates the importance of careful consideration of administration protocols required for successful delivery of cell suspensions, according to their nature and cellular responses post-ejection.
Keywords: NIH 3T3; apoptosis; cell injection; cell therapies; viability.
© 2015 The Authors Journal of Pharmacy and Pharmacology published by John Wiley & Sons Ltd on behalf of Royal Pharmaceutical Society.
Figures



References
-
- Freed CR et al Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 2001; 10: 710–719. - PubMed
-
- Olanow CW et al A double‐blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 2003; 3: 403–414. - PubMed
-
- Bang OY et al Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005; 6: 874–882. - PubMed
-
- Kondziolka D et al Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurgery 2005; 1: 38–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

