Placental Hofbauer cells assemble and sequester HIV-1 in tetraspanin-positive compartments that are accessible to broadly neutralizing antibodies
- PMID: 25623930
- PMCID: PMC4308659
- DOI: 10.7448/IAS.18.1.19385
Placental Hofbauer cells assemble and sequester HIV-1 in tetraspanin-positive compartments that are accessible to broadly neutralizing antibodies
Abstract
Introduction: Within monocyte-derived macrophages, HIV-1 accumulates in intracellular virus-containing compartments (VCCs) that are inaccessible to the external environment, which implicate these cells as latently infected HIV-1 reservoirs. During mother-to-child transmission of HIV-1, human placental macrophages (Hofbauer cells (HCs)) are viral targets, and have been shown to be infected in vivo and sustain low levels of viral replication in vitro; however, the risk of in utero transmission is less than 7%. The role of these primary macrophages as viral reservoirs is largely undefined. The objective of this study is to define potential sites of viral assembly, accumulation and neutralization in HCs given the pivotal role of the placenta in preventing HIV-1 infection in the mother-infant dyad.
Methods: Term placentae from 20 HIV-1 seronegative women were obtained following caesarian section. VCCs were evaluated by 3D confocal and electron microscopy. Colocalization R values (Pearson's correlation) were quantified with colocalization module of Volocity 5.2.1. Replication kinetics and neutralization studies were evaluated using p24 ELISA.
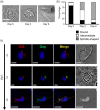
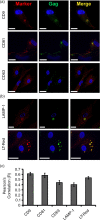
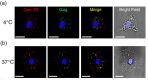
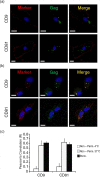
Results: We demonstrate that primary HCs assemble and sequester HIV-1(BaL) in intracellular VCCs, which are enriched in endosomal/lysosomal markers, including CD9, CD81, CD63 and LAMP-1. Following infection, we observed HIV-1 accumulation in potentially acidic compartments, which stained intensely with Lysotracker-Red. Remarkably, these compartments are readily accessible via the cell surface and can be targeted by exogenously applied small molecules and HIV-1-specific broadly neutralizing antibodies. In addition, broadly neutralizing antibodies (4E10 and VRC01) limited viral replication by HIV-1-infected HCs, which may be mediated by FcγRI.
Conclusions: These findings suggest that placental HCs possess intrinsic adaptations facilitating unique sequestration of HIV-1, and may serve as a protective viral reservoir to permit viral neutralization and/or antiretroviral drug entry in utero.
Keywords: HIV-1; Hofbauer cells; MTCT; VCCs; bNAbs; placenta.
Figures

Similar articles
-
Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines.Retrovirology. 2012 Dec 5;9:101. doi: 10.1186/1742-4690-9-101. Retrovirology. 2012. PMID: 23217137 Free PMC article.
-
CD4+ T cells facilitate replication of primary HIV-1 strains in macrophages and formation of macrophage internal virus-containing compartments.J Virol. 2025 Apr 15;99(4):e0018225. doi: 10.1128/jvi.00182-25. Epub 2025 Mar 25. J Virol. 2025. PMID: 40130873 Free PMC article.
-
Macrophage internal HIV-1 is protected from neutralizing antibodies.J Virol. 2012 Mar;86(5):2826-36. doi: 10.1128/JVI.05915-11. Epub 2011 Dec 28. J Virol. 2012. PMID: 22205742 Free PMC article.
-
HIV-1 at the placenta: immune correlates of protection and infection.Curr Opin Infect Dis. 2016 Jun;29(3):248-55. doi: 10.1097/QCO.0000000000000267. Curr Opin Infect Dis. 2016. PMID: 27027245 Review.
-
Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection.Arch Gynecol Obstet. 2017 Jun;295(6):1361-1368. doi: 10.1007/s00404-017-4361-5. Epub 2017 Apr 11. Arch Gynecol Obstet. 2017. PMID: 28396992 Free PMC article. Review.
Cited by
-
Vaccine-Derived Neutralizing Antibodies to the Human Cytomegalovirus gH/gL Pentamer Potently Block Primary Cytotrophoblast Infection.J Virol. 2015 Dec;89(23):11884-98. doi: 10.1128/JVI.01701-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378171 Free PMC article.
-
Distinct immune responses to HIV and CMV in Hofbauer cells across gestation highlight evolving placental immune dynamics.bioRxiv [Preprint]. 2024 Nov 22:2024.11.21.624730. doi: 10.1101/2024.11.21.624730. bioRxiv. 2024. PMID: 39605623 Free PMC article. Preprint.
-
Placental Macrophage (Hofbauer Cell) Responses to Infection During Pregnancy: A Systematic Scoping Review.Front Immunol. 2022 Feb 11;12:756035. doi: 10.3389/fimmu.2021.756035. eCollection 2021. Front Immunol. 2022. PMID: 35250964 Free PMC article.
-
Human Cytomegalovirus Enhances Placental Susceptibility and Replication of Human Immunodeficiency Virus Type 1 (HIV-1), Which May Facilitate In Utero HIV-1 Transmission.J Infect Dis. 2018 Sep 22;218(9):1464-1473. doi: 10.1093/infdis/jiy327. J Infect Dis. 2018. PMID: 29860306 Free PMC article.
-
Zika Fetal Neuropathogenesis: Etiology of a Viral Syndrome.PLoS Negl Trop Dis. 2016 Aug 25;10(8):e0004877. doi: 10.1371/journal.pntd.0004877. eCollection 2016 Aug. PLoS Negl Trop Dis. 2016. PMID: 27560129 Free PMC article. Review.
References
-
- Simister NE. Human placental Fc receptors and the trapping of immune complexes. Vaccine. 1998;16(14–15):1451–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

