Thirst driving and suppressing signals encoded by distinct neural populations in the brain
- PMID: 25624099
- PMCID: PMC4401619
- DOI: 10.1038/nature14108
Thirst driving and suppressing signals encoded by distinct neural populations in the brain
Abstract
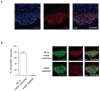
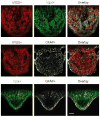
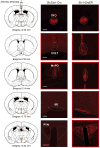
Thirst is the basic instinct to drink water. Previously, it was shown that neurons in several circumventricular organs of the hypothalamus are activated by thirst-inducing conditions. Here we identify two distinct, genetically separable neural populations in the subfornical organ that trigger or suppress thirst. We show that optogenetic activation of subfornical organ excitatory neurons, marked by the expression of the transcription factor ETV-1, evokes intense drinking behaviour, and does so even in fully water-satiated animals. The light-induced response is highly specific for water, immediate and strictly locked to the laser stimulus. In contrast, activation of a second population of subfornical organ neurons, marked by expression of the vesicular GABA transporter VGAT, drastically suppresses drinking, even in water-craving thirsty animals. These results reveal an innate brain circuit that can turn an animal's water-drinking behaviour on and off, and probably functions as a centre for thirst control in the mammalian brain.
Figures

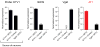






References
-
- McKinley MJ, et al. The sensory circumventricular organs of the mammalian brain. Advances in anatomy, embryology, and cell biology. 2003;172:1–122. - PubMed
-
- Young JK. Hunger, Thirst, Sex, and Sleep: How the Brain Controls Our Passions. Rowman & Littlefield Publishers, Inc; 2012.
-
- Daniels D, Fluharty SJ. Salt appetite: a neurohormonal viewpoint. Physiology & behavior. 2004;81:319–337. - PubMed
-
- Geerling JC, Loewy AD. Central regulation of sodium appetite. Experimental physiology. 2008;93:177–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

