Phase III, randomized, double-blind, multicenter trial comparing orteronel (TAK-700) plus prednisone with placebo plus prednisone in patients with metastatic castration-resistant prostate cancer that has progressed during or after docetaxel-based therapy: ELM-PC 5
- PMID: 25624429
- PMCID: PMC4879718
- DOI: 10.1200/JCO.2014.56.5119
Phase III, randomized, double-blind, multicenter trial comparing orteronel (TAK-700) plus prednisone with placebo plus prednisone in patients with metastatic castration-resistant prostate cancer that has progressed during or after docetaxel-based therapy: ELM-PC 5
Abstract
Purpose: Orteronel (TAK-700) is an investigational, nonsteroidal, reversible, selective 17,20-lyase inhibitor. This study examined orteronel in patients with metastatic castration-resistant prostate cancer that progressed after docetaxel therapy.
Patients and methods: In our study, 1,099 men were randomly assigned in a 2:1 schedule to receive orteronel 400 mg plus prednisone 5 mg twice daily or placebo plus prednisone 5 mg twice daily, stratified by region (Europe, North America [NA], and non-Europe/NA) and Brief Pain Inventory-Short Form worst pain score. Primary end point was overall survival (OS). Key secondary end points (radiographic progression-free survival [rPFS], ≥ 50% decrease of prostate-specific antigen [PSA50], and pain response at 12 weeks) were to undergo statistical testing only if the primary end point analysis was significant.
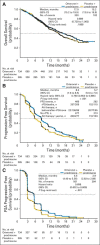
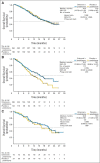
Results: The study was unblinded after crossing a prespecified OS futility boundary. The median OS was 17.0 months versus 15.2 months with orteronel-prednisone versus placebo-prednisone (hazard ratio [HR], 0.886; 95% CI, 0.739 to 1.062; P = .190). Improved rPFS was observed with orteronel-prednisone (median, 8.3 v 5.7 months; HR, 0.760; 95% CI, 0.653 to 0.885; P < .001). Orteronel-prednisone showed advantages over placebo-prednisone in PSA50 rate (25% v 10%, P < .001) and time to PSA progression (median, 5.5 v 2.9 months, P < .001) but not pain response rate (12% v 9%; P = .128). Adverse events (all grades) were generally more frequent with orteronel-prednisone, including nausea (42% v 26%), vomiting (36% v 17%), fatigue (29% v 23%), and increased amylase (14% v 2%).
Conclusion: Our study did not meet the primary end point of OS. Longer rPFS and a higher PSA50 rate with orteronel-prednisone indicate antitumor activity.
Trial registration: ClinicalTrials.gov NCT01193257.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures



Comment in
-
Failure of ELM-PC 5: an ineffective drug or an unfit end point?J Clin Oncol. 2015 Mar 1;33(7):679-81. doi: 10.1200/JCO.2014.59.4309. Epub 2015 Feb 2. J Clin Oncol. 2015. PMID: 25646190 No abstract available.
-
Prostate cancer: CYP17A1 inhibitor failure-lessons for future drug development.Nat Rev Urol. 2015 May;12(5):245-6. doi: 10.1038/nrurol.2015.66. Epub 2015 Mar 31. Nat Rev Urol. 2015. PMID: 25823374 No abstract available.
-
Reply to K. Lu.J Clin Oncol. 2015 Oct 1;33(28):3222-3. doi: 10.1200/JCO.2015.62.4692. Epub 2015 Jul 27. J Clin Oncol. 2015. PMID: 26215948 No abstract available.
-
Reply to K. Lu.J Clin Oncol. 2015 Oct 1;33(28):3222. doi: 10.1200/JCO.2015.62.4684. Epub 2015 Jul 27. J Clin Oncol. 2015. PMID: 26215954 No abstract available.
-
The Negative Is Not So Negative: What Do We Learn From Trials With Orteronel?J Clin Oncol. 2015 Oct 1;33(28):3221. doi: 10.1200/JCO.2015.62.3165. Epub 2015 Jul 27. J Clin Oncol. 2015. PMID: 26215959 No abstract available.
-
Re: Phase III, Randomized, Double-Blind, Multicenter Trial Comparing Orteronel (TAK-700) plus Prednisone with Placebo plus Prednisone in Patients with Metastatic Castration-Resistant Prostate Cancer that has Progressed during or after Docetaxel-Based Therapy: ELM-PC 5.J Urol. 2015 Oct;194(4):990. doi: 10.1016/j.juro.2015.07.010. Epub 2015 Jul 9. J Urol. 2015. PMID: 26382777 No abstract available.
References
-
- Huggins C, Hodges CV. Studies on prostatic cancer, I: The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–240. - PubMed
-
- Horwich A, Hugosson J, de Reijke T, et al. Prostate cancer: ESMO Consensus Conference Guidelines 2012. Ann Oncol. 2013;24:1141–1162. - PubMed
-
- Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. - PubMed
-
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

