Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma
- PMID: 25624430
- PMCID: PMC4348638
- DOI: 10.1200/JCO.2014.58.3401
Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma
Abstract
Purpose: The phosphoinositol-3 kinase (PI3K) pathway is frequently dysregulated in endometrial cancer (EC). Hormonal manipulation leads to response in some patients with EC, but resistance derived from PI3K pathway activation has been documented. Targeting mammalian target of rapamycin (mTOR) may overcome endocrine resistance. We conducted a two-institution phase II trial of everolimus and letrozole in women with recurrent EC.
Patients and methods: Patients were considered incurable, had measurable disease, and were treated with up to two prior cytotoxic regimens. Everolimus was administered orally at 10 mg daily and letrozole was administered orally at 2.5 mg daily. Each cycle consisted of 4 weeks of therapy. Patients were treated until progression, toxicity, or complete response (CR). The primary end point was the clinical benefit rate (CBR), which was defined as CR, partial response, or stable disease (≥ 16 weeks) by RECIST 1.0 criteria. Translational studies were performed to correlate biomarkers with response.
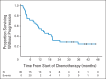
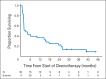
Results: Thirty-eight patients were enrolled (median age, 62 years; range, 24 to 82 years). Thirty-five patients were evaluable for response. The CBR was 40% (14 of 35 patients); the median number of cycles among responders was 15 (range, seven to 29 cycles). The confirmed objective response rate (RR) was 32% (11 of 35 patients; nine CRs and two partial responses; median, 15 cycles; range, eight to 29 cycles). Twenty percent of patients (seven of 35 patients) were taken off treatment after a prolonged CR and at the discretion of the treating clinician. None of the patients discontinued treatment as a result of toxicity. Serous histology was the best predictor of lack of response. Patients with endometrioid histology and CTNNB1 mutations responded well to everolimus and letrozole.
Conclusion: Everolimus plus letrozole results in a high CBR and RR in patients with recurrent EC. Further development of this combination in recurrent endometrioid EC is under way.
Trial registration: ClinicalTrials.gov NCT01068249.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Treatment of Recurrent Endometrial Carcinoma: Progress Toward a More Personalized Approach.J Clin Oncol. 2015 Oct 20;33(30):3516. doi: 10.1200/JCO.2015.61.4636. Epub 2015 Aug 3. J Clin Oncol. 2015. PMID: 26240220 No abstract available.
-
Reply to G. Bogani et al.J Clin Oncol. 2015 Oct 20;33(30):3516. doi: 10.1200/JCO.2015.62.5632. Epub 2015 Aug 3. J Clin Oncol. 2015. PMID: 26240222 No abstract available.
Similar articles
-
A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma.Cancer. 2010 Dec 1;116(23):5415-9. doi: 10.1002/cncr.25515. Epub 2010 Aug 2. Cancer. 2010. PMID: 20681032 Free PMC article. Clinical Trial.
-
Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer.J Clin Oncol. 2009 Jun 1;27(16):2630-7. doi: 10.1200/JCO.2008.18.8391. Epub 2009 Apr 20. J Clin Oncol. 2009. PMID: 19380449 Clinical Trial.
-
PIK3CA genotype and a PIK3CA mutation-related gene signature and response to everolimus and letrozole in estrogen receptor positive breast cancer.PLoS One. 2013;8(1):e53292. doi: 10.1371/journal.pone.0053292. Epub 2013 Jan 2. PLoS One. 2013. PMID: 23301057 Free PMC article. Clinical Trial.
-
A new era of improving progression-free survival with dual blockade in postmenopausal HR(+), HER2(-) advanced breast cancer.Cancer Treat Rev. 2015 Feb;41(2):94-104. doi: 10.1016/j.ctrv.2014.12.011. Epub 2014 Dec 30. Cancer Treat Rev. 2015. PMID: 25575443 Review.
-
Future directions in the treatment of hormone-sensitive advanced breast cancer: the RAD001 (Everolimus)-letrozole clinical program.Semin Oncol. 2006 Apr;33(2 Suppl 7):S18-25. doi: 10.1053/j.seminoncol.2006.03.024. Semin Oncol. 2006. PMID: 16730273 Review.
Cited by
-
Chemotherapy for Endometrial Cancer in Adjuvant and Advanced Disease Settings.Oncologist. 2016 Oct;21(10):1250-1259. doi: 10.1634/theoncologist.2016-0062. Epub 2016 Jul 13. Oncologist. 2016. PMID: 27412393 Free PMC article. Review.
-
Clinical Outcomes of Genotype-Matched Therapy for Recurrent Gynecological Cancers: A Single Institutional Experience.Healthcare (Basel). 2021 Oct 19;9(10):1395. doi: 10.3390/healthcare9101395. Healthcare (Basel). 2021. PMID: 34683075 Free PMC article.
-
The current state of molecular testing in the treatment of patients with solid tumors, 2019.CA Cancer J Clin. 2019 Jul;69(4):305-343. doi: 10.3322/caac.21560. Epub 2019 May 22. CA Cancer J Clin. 2019. PMID: 31116423 Free PMC article. Review.
-
Recent advances in endometrial cancer: a review of key clinical trials from 2015 to 2019.F1000Res. 2019 Jun 12;8:F1000 Faculty Rev-849. doi: 10.12688/f1000research.17408.1. eCollection 2019. F1000Res. 2019. PMID: 31231511 Free PMC article. Review.
-
Hormone Therapy plus mTOR Inhibitors in the Treatment of Endometrial Carcinoma.Oncol Hematol Rev. 2013;9(1):41-44. doi: 10.17925/ohr.2013.09.1.41. Oncol Hematol Rev. 2013. PMID: 25431800 Free PMC article.
References
-
- Catasus L, D'Angelo E, Pons C, et al. Expression profiling of 22 genes involved in the PI3K-AKT pathway identifies two subgroups of high-grade endometrial carcinomas with different molecular alterations. Mod Pathol. 2010;23:694–702. - PubMed
-
- Abe K, Abe T, Adachi I, et al. Observation of chi(c2) production in B meson decay. Phys Rev Lett. 2002;89 011803. - PubMed
-
- Mackay H, Welch S, Tsao MS, et al. Phase II study of oral ridaforolimus in patients with metastatic and/or locally advanced recurrent endometrial cancer. J Clin Oncol. 2011;(suppl):29. abstr 5013.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous