Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia
- PMID: 25624441
- PMCID: PMC4375304
- DOI: 10.1200/JCO.2014.59.4671
Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia
Abstract
Purpose: Mercaptopurine (MP) is the mainstay of curative therapy for acute lymphoblastic leukemia (ALL). We performed a genome-wide association study (GWAS) to identify comprehensively the genetic basis of MP intolerance in children with ALL.
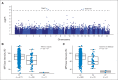
Patients and methods: The discovery GWAS and replication cohorts included 657 and 371 children from two prospective clinical trials. MP dose intensity was a marker for drug tolerance and toxicities and was defined as prescribed dose divided by the planned protocol dose during maintenance therapy; its association with genotype was evaluated using a linear mixed-effects model.
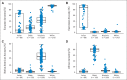
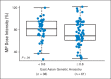
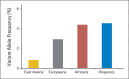
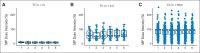
Results: MP dose intensity varied by race and ethnicity and was negatively correlated with East Asian genetic ancestry (P < .001). The GWAS revealed two genome-wide significant loci associated with dose intensity: rs1142345 in TPMT (Tyr240Cys, present in *3A and *3C variants; P = 8.6 × 10(-9)) and rs116855232 in NUDT15 (P = 8.8 × 10(-9)), with independent replication. Patients with TT genotype at rs116855232 were exquisitely sensitive to MP, with an average dose intensity of 8.3%, compared with those with TC and CC genotypes, who tolerated 63% and 83.5% of the planned dose, respectively. The NUDT15 variant was most common in East Asians and Hispanics, rare in Europeans, and not observed in Africans, contributing to ancestry-related differences in MP tolerance. Of children homozygous for either TPMT or NUDT15 variants or heterozygous for both, 100% required ≥ 50% MP dose reduction, compared with only 7.7% of others.
Conclusion: We describe a germline variant in NUDT15 strongly associated with MP intolerance in childhood ALL, which may have implications for treatment individualization in this disease.
Trial registration: ClinicalTrials.gov NCT00137111 NCT00268528.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures





Comment in
-
Heritage-specific mechanisms for cancer adverse reactions: one gene does not explain the world.J Clin Oncol. 2015 Apr 10;33(11):1230-1. doi: 10.1200/JCO.2014.60.1740. Epub 2015 Mar 9. J Clin Oncol. 2015. PMID: 25753435 No abstract available.
References
-
- Koren G, Ferrazini G, Sulh H, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. 1990;323:17–21. - PubMed
-
- Relling MV, Hancock ML, Boyett JM, et al. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999;93:2817–2823. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- GM92666/GM/NIGMS NIH HHS/United States
- R01 CA096670/CA/NCI NIH HHS/United States
- CA156449/CA/NCI NIH HHS/United States
- R37 CA036401/CA/NCI NIH HHS/United States
- CA36401/CA/NCI NIH HHS/United States
- U10 CA095861/CA/NCI NIH HHS/United States
- CA095861/CA/NCI NIH HHS/United States
- RC4 CA156449/CA/NCI NIH HHS/United States
- CA21765/CA/NCI NIH HHS/United States
- CA098543/CA/NCI NIH HHS/United States
- UG1 CA189955/CA/NCI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- U10 CA098543/CA/NCI NIH HHS/United States
- R01 CA036401/CA/NCI NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
- CA096670/CA/NCI NIH HHS/United States
- U10 CA180886/CA/NCI NIH HHS/United States
- U01 GM092666/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

