Gating pore currents are defects in common with two Nav1.5 mutations in patients with mixed arrhythmias and dilated cardiomyopathy
- PMID: 25624448
- PMCID: PMC4306709
- DOI: 10.1085/jgp.201411304
Gating pore currents are defects in common with two Nav1.5 mutations in patients with mixed arrhythmias and dilated cardiomyopathy
Abstract
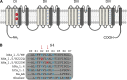
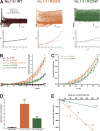
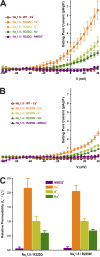
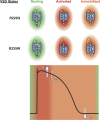
The gating pore current, also called omega current, consists of a cation leak through the typically nonconductive voltage-sensor domain (VSD) of voltage-gated ion channels. Although the study of gating pore currents has refined our knowledge of the structure and the function of voltage-gated ion channels, their implication in cardiac disorders has not been established. Two Na(v)1.5 mutations (R222Q and R225W) located in the VSD are associated with atypical clinical phenotypes involving complex arrhythmias and dilated cardiomyopathy. Using the patch-clamp technique, in silico mutagenesis, and molecular dynamic simulations, we tested the hypothesis that these two mutations may generate gating pore currents, potentially accounting for their clinical phenotypes. Our findings suggest that the gating pore current generated by the R222Q and R225W mutations could constitute the underlying pathological mechanism that links Na(v)1.5 VSD mutations with human cardiac arrhythmias and dilatation of cardiac chambers.
© 2015 Moreau et al.
Figures




References
-
- Bezzina C.R., Rook M.B., Groenewegen W.A., Herfst L.J., van der Wal A.C., Lam J., Jongsma H.J., Wilde A.A., and Mannens M.M.. 2003. Compound heterozygosity for mutations (W156X and R225W) in SCN5A associated with severe cardiac conduction disturbances and degenerative changes in the conduction system. Circ. Res. 92:159–168 10.1161/01.RES.0000052672.97759.36 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

