Membranes serve as allosteric activators of phospholipase A2, enabling it to extract, bind, and hydrolyze phospholipid substrates
- PMID: 25624474
- PMCID: PMC4330758
- DOI: 10.1073/pnas.1424651112
Membranes serve as allosteric activators of phospholipase A2, enabling it to extract, bind, and hydrolyze phospholipid substrates
Abstract
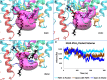
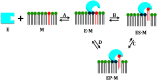
Defining the molecular details and consequences of the association of water-soluble proteins with membranes is fundamental to understanding protein-lipid interactions and membrane functioning. Phospholipase A2 (PLA2) enzymes, which catalyze the hydrolysis of phospholipid substrates that compose the membrane bilayers, provide the ideal system for studying protein-lipid interactions. Our study focuses on understanding the catalytic cycle of two different human PLA2s: the cytosolic Group IVA cPLA2 and calcium-independent Group VIA iPLA2. Computer-aided techniques guided by deuterium exchange mass spectrometry data, were used to create structural complexes of each enzyme with a single phospholipid substrate molecule, whereas the substrate extraction process was studied using steered molecular dynamics simulations. Molecular dynamic simulations of the enzyme-substrate-membrane systems revealed important information about the mechanisms by which these enzymes associate with the membrane and then extract and bind their phospholipid substrate. Our data support the hypothesis that the membrane acts as an allosteric ligand that binds at the allosteric site of the enzyme's interfacial surface, shifting its conformation from a closed (inactive) state in water to an open (active) state at the membrane interface.
Keywords: DXMS; GIVA cPLA2; GVIA iPLA2; MD simulations; PAPC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



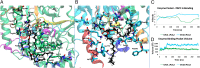
References
-
- Masuda S, et al. Various secretory phospholipase A2 enzymes are expressed in rheumatoid arthritis and augment prostaglandin production in cultured synovial cells. FEBS J. 2005;272(3):655–672. - PubMed
-
- Rosenson RS, Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur Heart J. 2012;33(23):2899–2909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

