Src phosphorylation converts Mdm2 from a ubiquitinating to a neddylating E3 ligase
- PMID: 25624478
- PMCID: PMC4330765
- DOI: 10.1073/pnas.1416656112
Src phosphorylation converts Mdm2 from a ubiquitinating to a neddylating E3 ligase
Abstract
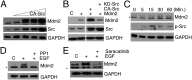
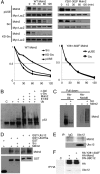
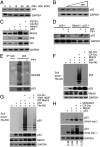
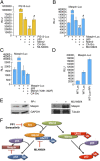
Murine double minute-2 protein (Mdm2) is a multifaceted phosphorylated protein that plays a role in regulating numerous proteins including the tumor suppressor protein p53. Mdm2 binds to and is involved in conjugating either ubiquitin or Nedd8 (Neural precursor cell expressed, developmentally down-regulated 8) to p53. Although regulation of the E3 ubiquitin activity of Mdm2 has been investigated, regulation of the neddylating activity of Mdm2 remains to be defined. Here we show that activated c-Src kinase phosphorylates Y281 and Y302 of Mdm2, resulting in an increase in Mdm2 stability and its association with Ubc12, the E2 enzyme of the neddylating complex. Mdm2-dependent Nedd8 conjugation of p53 results in transcriptionally inactive p53, a process that is reversed with a small molecule inhibitor to either Src or Ubc12. Thus, our studies reveal how Mdm2 may neutralize and elevate p53 in actively proliferating cells and also provides a rationale for using therapies that target the Nedd8 pathway in wild-type p53 tumors.
Keywords: Mdm2; Nedd8; Src.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53.EMBO J. 1999 Jan 4;18(1):22-7. doi: 10.1093/emboj/18.1.22. EMBO J. 1999. PMID: 9878046 Free PMC article.
-
Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity.Cell. 2004 Jul 9;118(1):83-97. doi: 10.1016/j.cell.2004.06.016. Cell. 2004. PMID: 15242646
-
NUB1 promotes cytoplasmic localization of p53 through cooperation of the NEDD8 and ubiquitin pathways.Oncogene. 2010 Apr 15;29(15):2252-61. doi: 10.1038/onc.2009.494. Epub 2010 Jan 25. Oncogene. 2010. PMID: 20101219
-
Regulation of the p53 pathway by ubiquitin and related proteins.Int J Biochem Cell Biol. 2010 Oct;42(10):1618-21. doi: 10.1016/j.biocel.2010.06.011. Epub 2010 Jun 17. Int J Biochem Cell Biol. 2010. PMID: 20601087 Review.
-
[Progress in regulation of activity and stability of ubiquitin protein ligase MDM2].Yi Chuan. 2009 Oct;31(10):993-8. doi: 10.3724/sp.j.1005.2009.00993. Yi Chuan. 2009. PMID: 19840920 Review. Chinese.
Cited by
-
Dysregulation of ubiquitin ligases in cancer.Drug Resist Updat. 2015 Nov;23:1-11. doi: 10.1016/j.drup.2015.09.001. Epub 2015 Sep 28. Drug Resist Updat. 2015. PMID: 26690337 Free PMC article. Review.
-
Effects of neddylation on viral infection: an overview.Arch Virol. 2023 Dec 11;169(1):6. doi: 10.1007/s00705-023-05930-3. Arch Virol. 2023. PMID: 38081982 Review.
-
MDM2 inhibition is synthetic lethal with PTEN loss in colorectal cancer cells via the p53-dependent mechanism.Int J Biol Sci. 2023 Jul 9;19(11):3544-3557. doi: 10.7150/ijbs.82566. eCollection 2023. Int J Biol Sci. 2023. PMID: 37496993 Free PMC article.
-
Neddylation: A Versatile Pathway Takes on Chronic Liver Diseases.Front Med (Lausanne). 2020 Oct 19;7:586881. doi: 10.3389/fmed.2020.586881. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33195347 Free PMC article. Review.
-
Induction of the Mdm2 gene and protein by kinase signaling pathways is repressed by the pVHL tumor suppressor.Proc Natl Acad Sci U S A. 2024 Jul 30;121(31):e2400935121. doi: 10.1073/pnas.2400935121. Epub 2024 Jul 24. Proc Natl Acad Sci U S A. 2024. PMID: 39047034 Free PMC article.
References
-
- Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4(6):470–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

