Structural and biochemical studies of HCMV gH/gL/gO and Pentamer reveal mutually exclusive cell entry complexes
- PMID: 25624487
- PMCID: PMC4330774
- DOI: 10.1073/pnas.1424818112
Structural and biochemical studies of HCMV gH/gL/gO and Pentamer reveal mutually exclusive cell entry complexes
Abstract
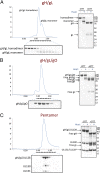
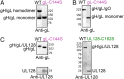
Human cytomegalovirus (HCMV) is a major cause of morbidity and mortality in transplant patients and the leading viral cause of birth defects after congenital infection. The glycoprotein complexes gH/gL/gO and gH/gL/UL128/UL130/UL131A (Pentamer) are key targets of the human humoral response against HCMV and are required for HCMV entry into fibroblasts and endothelial/epithelial cells, respectively. We expressed and characterized soluble forms of gH/gL, gH/gL/gO, and Pentamer. Mass spectrometry and mutagenesis analysis revealed that gL-Cys144 forms disulfide bonds with gO-Cys351 in gH/gL/gO and with UL128-Cys162 in the Pentamer. Notably, Pentamer harboring the UL128-Cys162Ser/gL-Cys144Ser mutations had impaired syncytia formation and reduced interference of HCMV entry into epithelial cells. Electron microscopy analysis showed that HCMV gH/gL resembles HSV gH/gL and that gO and UL128/UL130/UL131A bind to the same site at the gH/gL N terminus. These data are consistent with gH/gL/gO and Pentamer forming mutually exclusive cell entry complexes and reveal the overall location of gH/gL-, gH/gL/gO-, and Pentamer-specific neutralizing antibody binding sites. Our results provide, to our knowledge, the first structural view of gH/gL/gO and Pentamer supporting the development of vaccines and antibody therapeutics against HCMV.
Keywords: HCMV; Pentamer complex; gH/gL/gO; human cytomegalovirus; virus entry.
Conflict of interest statement
Conflict of interest statement: The sponsor is a full-time employee of Novartis Vaccines, as are S.C., R.G., S.W.B., N.N., E.C.S., and A.C.
Figures




References
-
- Ho M. Epidemiology of cytomegalovirus infections. Rev Infect Dis. 1990;12(Suppl 7):S701–S710. - PubMed
-
- Britt W. Manifestations of human cytomegalovirus infection: Proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol. 2008;325:417–470. - PubMed
-
- Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. National Vaccine Advisory Committee Vaccine development to prevent cytomegalovirus disease: Report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39(2):233–239. - PubMed
-
- Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

