Therapeutic mechanisms of high-frequency stimulation in Parkinson's disease and neural restoration via loop-based reinforcement
- PMID: 25624501
- PMCID: PMC4330724
- DOI: 10.1073/pnas.1406549111
Therapeutic mechanisms of high-frequency stimulation in Parkinson's disease and neural restoration via loop-based reinforcement
Abstract
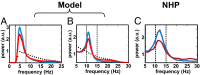
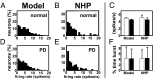
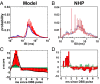
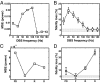
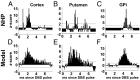
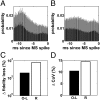
High-frequency deep brain stimulation (HFS) is clinically recognized to treat parkinsonian movement disorders, but its mechanisms remain elusive. Current hypotheses suggest that the therapeutic merit of HFS stems from increasing the regularity of the firing patterns in the basal ganglia (BG). Although this is consistent with experiments in humans and animal models of Parkinsonism, it is unclear how the pattern regularization would originate from HFS. To address this question, we built a computational model of the cortico-BG-thalamo-cortical loop in normal and parkinsonian conditions. We simulated the effects of subthalamic deep brain stimulation both proximally to the stimulation site and distally through orthodromic and antidromic mechanisms for several stimulation frequencies (20-180 Hz) and, correspondingly, we studied the evolution of the firing patterns in the loop. The model closely reproduced experimental evidence for each structure in the loop and showed that neither the proximal effects nor the distal effects individually account for the observed pattern changes, whereas the combined impact of these effects increases with the stimulation frequency and becomes significant for HFS. Perturbations evoked proximally and distally propagate along the loop, rendezvous in the striatum, and, for HFS, positively overlap (reinforcement), thus causing larger poststimulus activation and more regular patterns in striatum. Reinforcement is maximal for the clinically relevant 130-Hz stimulation and restores a more normal activity in the nuclei downstream. These results suggest that reinforcement may be pivotal to achieve pattern regularization and restore the neural activity in the nuclei downstream and may stem from frequency-selective resonant properties of the loop.
Keywords: Parkinson’s disease; basal ganglia; deep brain stimulation; reinforcement; thalamus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- The Deep-Brain Stimulation for Parkinson’s Disease Study Group Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345(13):956–963. - PubMed
-
- Durif F, Lemaire JJ, Debilly B, Dordain G. Long-term follow-up of globus pallidus chronic stimulation in advanced Parkinson’s disease. Mov Disord. 2002;17(4):803–807. - PubMed
-
- Rodriguez-Oroz MC, et al. Bilateral deep brain stimulation in Parkinson’s disease: A multicentre study with 4 years follow-up. Brain. 2005;128(Pt 10):2240–2249. - PubMed
-
- Moro E, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord. 2010;25(5):578–586. - PubMed
-
- Montgomery EB, Jr, Gale JT. Mechanisms of action of deep brain stimulation (DBS) Neurosci Biobehav Rev. 2008;32(3):388–407. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

