The expanding realm of endovascular neurosurgery: flow diversion for cerebral aneurysm management
- PMID: 25624975
- PMCID: PMC4300059
- DOI: 10.14797/mdcj-10-4-214
The expanding realm of endovascular neurosurgery: flow diversion for cerebral aneurysm management
Abstract
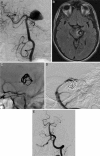
The worldwide prevalence of intracranial aneurysms is estimated to be between 5% and 10%, with some demographic variance. Subarachnoid hemorrhage secondary to ruptured intracranial aneurysm results in devastating neurological outcomes, leaving the majority of victims dead or disabled. Surgical clipping of intracranial aneurysms remained the definitive mode of treatment until Guglielmi detachable coils were introduced in the 1990s. This revolutionary innovation led to the recognition of neurointervention/neuroendovascular surgery as a bona fide option for intracranial aneurysms. Constant evolution of endovascular devices and techniques supported by several prospective randomized trials has catapulted the endovascular treatment of intracranial aneurysms to its current status as the preferred treatment modality for most ruptured and unruptured intracranial aneurysms. We are slowly transitioning from the era of coils to the era of flow diverters. Flow-diversion technology and techniques have revolutionized the treatment of wide-necked, giant, and fusiform aneurysms, where the results of microsurgery or conventional neuroendovascular strategies have traditionally been dismal. Although the Pipeline Embolization Device (ev3-Covidien, Irvine, CA) is the only flow-diversion device approved by the Food and Drug Administration for use in the United States, others are commercially available in Europe and South America, including the Silk (Balt Extrusion, Montmorency, France), Flow-Redirection Endoluminal Device (FRED; MicroVention, Tustin, CA), Surpass (Stryker, Kalamazoo, MI), and p64 (Phenox, Bochum, Germany). Improvements in technology and operator experience and the encouraging results of clinical trials have led to broader acceptance for the use of these devices in cerebral aneurysm management. Continued innovation and refinement of endovascular devices and techniques will inevitably improve technical success rates, reduce procedure-related complications, and broaden the endovascular therapeutic spectrum for varied aneurysm morphology.
Keywords: flow diverter; intracranial aneurysm; pipeline embolization device; subarachnoid hemorrhage.
Figures


References
-
- Caranci F, Briganti F, Cirillo L, Leonardi M, Muto M. Epidemiology and genetics of intracranial aneurysms. Eur J Radiol. 2013 Oct;82(10):1598–605. - PubMed
-
- Morita A, Fujiwara S, Hashi K, Ohtsu H, Kirino T. Risk of rupture associated with intact cerebral aneurysms in the Japanese population: a systematic review of the literature from Japan. J Neurosurg. 2005 Apr;102(4):601–6. - PubMed
-
- Ingall T, Asplund K, Mähönen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke. 2000 May;31(5):1054–61. - PubMed
-
- Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012 Jun;43(6):1711–37. et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

