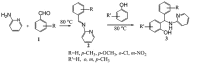Solvent-free preparation of novel 2-[phenyl (pyridine-2-ylamino) methyl] phenols as pseudo-betti processor for natural products
- PMID: 25625053
- PMCID: PMC4302402
- DOI: 10.17795/jjnpp-17808
Solvent-free preparation of novel 2-[phenyl (pyridine-2-ylamino) methyl] phenols as pseudo-betti processor for natural products
Abstract
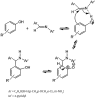
Background: 2-Aminopyridine and benzaldehydes mixture readily reacted with phenols at 80°C without any solvents to produce novel 2-[phenyl (pyridine-2-yl amino (methyl] phenol derivatives as pseudo-Betti products in good to high yields. These compounds are efficient processor for synthesis of the natural products.
Objectives: We decided to report the synthesis of a series of novel N-heteroaryl-arylmethyl phenols via a simple three-component, one-pot method, using aromatic aldehydes, heteroaryl amines, and phenols in the absence of any acid catalysts and under solvent-free conditions.
Materials and methods: All starting materials were purchased from Merck and Aldrich companies. The IR spectra were recorded on a Perkin-Elmer RXI infrared spectrometer.
Results: The reaction is convenient, operationally simple, proceeds quickly, and does not need solvents or expensive starting materials. The structures of the products were characterized by their spectral (1H NMR and IR) data.
Conclusions: We have developed a new, simple, and efficient method for one-pot aminoalkylation of active phenol compounds with various imines prepared from 2-aminopyrimidine and benzaldehydes in good to high yields (40%-97%).
Keywords: 2-Amino pyridine; Base; Phenols; Pseudo-Betti; Solvent-Free.
Figures
Similar articles
-
Citrus Juice: Green and Natural Catalyst for the Solvent-free Silica Supported Synthesis of β-Enaminones Using Grindstone Technique.Comb Chem High Throughput Screen. 2018;21(1):19-25. doi: 10.2174/1386207321666180102115733. Comb Chem High Throughput Screen. 2018. PMID: 29295688
-
Green Synthesis of Fused Chromeno-pyrazolo-phthalazine Derivatives with Silicasupported Bismuth Nitrate under Solvent-free Conditions.Curr Org Synth. 2021;18(8):854-861. doi: 10.2174/1570179417666201208112520. Curr Org Synth. 2021. PMID: 33292122
-
A Simple and Efficient Synthesis of 4-Arylacridinediones and 6-Aryldiindeno[1,2-b:2,1-e]pyridinediones using CuI Nanoparticles as Catalyst under Solvent-Free Conditions.Comb Chem High Throughput Screen. 2017;20(9):773-780. doi: 10.2174/1386207320666171002123027. Comb Chem High Throughput Screen. 2017. PMID: 28969547
-
Recent advances in the synthesis and synthetic applications of Betti base (aminoalkylnaphthol) and bis-Betti base derivatives.RSC Adv. 2019 Jun 11;9(32):18467-18497. doi: 10.1039/c9ra02813g. eCollection 2019 Jun 10. RSC Adv. 2019. PMID: 35515249 Free PMC article. Review.
-
Recent advances in the green synthesis of Betti bases and their applications: a review.Mol Divers. 2023 Feb;27(1):543-569. doi: 10.1007/s11030-022-10427-3. Epub 2022 Apr 21. Mol Divers. 2023. PMID: 35449388 Review.
Cited by
-
Recent advances in bio-based polybenzoxazines as an interesting adhesive coating.RSC Adv. 2023 Jul 3;13(29):19817-19835. doi: 10.1039/d3ra03514j. eCollection 2023 Jun 29. RSC Adv. 2023. PMID: 37404316 Free PMC article. Review.
References
-
- Seebach D, Matthews J. eptides: a surprise at every turn. Chem Commun. 1997;21:2015–22.
-
- Wang Y, Izawa T, Kobayashi S, Ohno M. Stereo-controlled synthesis of (+)-negamycin from an acyclic homoallylamineby 1,3-asymmetric induction. J Am Chem Soc. 1982;104:6465–6.
-
- Knapp S. Synthesis of complex nucleoside antibiotics,. Chem Rev. 1995;95:1859–76.
-
- Stokker GE, Deana AA, deSolms SJ, Schultz EM, Smith RL, Cragoe EJ, Jr., et al. 2-(Aminomethyl)phenols, a new class of saluretic agents. 1. Effects of nuclear substitution. J Med Chem. 1980;23(12):1414–27. - PubMed
-
- Heydenreich M, Koch A, Klod S, Szatmari I, Fulop F, Kleinpeter E. Synthesis and conformational analysis of napht[10,20:5,6][1,3]oxazino [3,2-c][1,3]benzoxazine and napht[10,205,6][1,3]oxazino[3,4-c][1,3]benzoxazine derivatives. Tetrahedron. 2006;62:1081–110891.
LinkOut - more resources
Full Text Sources