Rapid microscopy and use of vital dyes: potential to determine viability of Cryptococcus neoformans in the clinical laboratory
- PMID: 25625210
- PMCID: PMC4308066
- DOI: 10.1371/journal.pone.0117186
Rapid microscopy and use of vital dyes: potential to determine viability of Cryptococcus neoformans in the clinical laboratory
Abstract
Background: Cryptococcus neoformans is the commonest cause of fungal meningitis, with a substantial mortality despite appropriate therapy. Quantitative culture of cryptococci in cerebrospinal fluid (CSF) during antifungal therapy is of prognostic value and has therapeutic implications, but is slow and not practicable in many resource-poor countries.
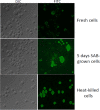
Methods: We piloted two rapid techniques for quantifying viable cryptococci using mixtures of live and heat-killed cryptococci cultured in vitro: (i) quantitative microscopy with exclusion staining using trypan blue dye, and (ii) flow cytometry, using the fluorescent dye 2'-7'-Bis-(2-carboxyethyl)-5-(6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM). Results were compared with standard quantitative cryptococcal cultures. Quantitative microscopy was also performed on cerebrospinal fluid (CSF) samples.
Results: Both microscopy and flow cytometry distinguished between viable and non-viable cryptococci. Cell counting (on log scale) by microscopy and by quantitative culture were significantly linearly associated (p<0.0001) and Bland-Altman analysis showed a high level of agreement. Proportions of viable cells (on logit scale), as detected by flow cytometry were significantly linearly associated with proportions detected by microscopy (p<0.0001) and Bland-Altman analysis showed a high level of agreement.
Conclusions: Direct microscopic examination of trypan blue-stained cryptococci and flow-cytometric assessment of BCECF-AM-stained cryptococci were in good agreement with quantitative cultures. These are promising strategies for rapid determination of the viability of cryptococci, and should be investigated in clinical practice.
Conflict of interest statement
Figures




References
-
- Brouwer AE, Teparrukkul P, Pinpraphaporn S, Larsen RA, Chierakul W, et al. (2005) Baseline Correlation and Comparative Kinetics of Cerebrospinal Fluid Colony-Forming Unit Counts and Antigen Titers in Cryptococcal Meningitis. Journal of Infectious Diseases 192: 681–684. - PubMed
-
- Robinson PA, Bauer M, Leal MAE, Evans SG, Holtom PD, et al. (1999) Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin Infect Dis 28: 82–92. - PubMed
-
- Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, et al. (2004) Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. The Lancet 363: 1764–1767. - PubMed
-
- Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, et al. (2009) Independent Association between Rate of Clearance of Infection and Clinical Outcome of HIV- Associated Cryptococcal Meningitis: Analysis of a Combined Cohort of 262 Patients. Clinical Infectious Diseases 49: 702–709. 10.1086/604716 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

