Lipid abnormalities in alpha/beta2-syntrophin null mice are independent from ABCA1
- PMID: 25625330
- PMCID: PMC4363173
- DOI: 10.1016/j.bbalip.2015.01.012
Lipid abnormalities in alpha/beta2-syntrophin null mice are independent from ABCA1
Abstract
The syntrophins alpha (SNTA) and beta 2 (SNTB2) are molecular adaptor proteins shown to stabilize ABCA1, an essential regulator of HDL cholesterol. Furthermore, SNTB2 is involved in glucose stimulated insulin release. Hyperglycemia and dyslipidemia are characteristic features of the metabolic syndrome, a serious public health problem with rising prevalence. Therefore, it is important to understand the role of the syntrophins herein. Mice deficient for both syntrophins (SNTA/B2-/-) have normal insulin and glucose tolerance, hepatic ABCA1 protein and cholesterol. When challenged with a HFD, wild type and SNTA/B2-/- mice have similar weight gain, adiposity, serum and liver triglycerides. Hepatic ABCA1, serum insulin and insulin sensitivity are normal while glucose tolerance is impaired. Liver cholesterol is reduced, and expression of SREBP2 and HMG-CoA-R is increased in the knockout mice. Scavenger receptor-BI (SR-BI) protein is strongly diminished in the liver of SNTA/B2-/- mice while SR-BI binding protein NHERF1 is not changed and PDZK1 is even induced. Knock-down of SNTA, SNTB2 or both has no effect on hepatocyte SR-BI and PDZK1 proteins. Further, SR-BI levels are not reduced in brown adipose tissue of SNTA/B2-/- mice excluding that syntrophins directly stabilize SR-BI. SR-BI stability is regulated by MAPK and phosphorylated ERK2 is induced in the liver of the knock-out mice. Blockage of ERK activity upregulates hepatocyte SR-BI showing that increased MAPK activity contributes to low SR-BI. Sphingomyelin which is well described to regulate cholesterol metabolism is reduced in the liver and serum of the knock-out mice while the size of serum lipoproteins is not affected. Current data exclude a major function of these syntrophins in ABCA1 activity and insulin release but suggest a role in regulating glucose uptake, ERK and SR-BI levels, and sphingomyelin metabolism in obesity.
Keywords: Glucose tolerance test; Insulin; Liver; Scavenger receptor B-I; Sphingomyelin.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures






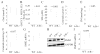

References
-
- Haffner SM. Relationship of metabolic risk factors and development of cardiovascular disease and diabetes. Obesity (Silver Spring) 2006;14(Suppl 3):121S–127S. - PubMed
-
- Hjermann I. The metabolic cardiovascular syndrome: syndrome X, Reaven’s syndrome, insulin resistance syndrome, atherothrombogenic syndrome. Journal of cardiovascular pharmacology. 1992;20(Suppl 8):S5–10. - PubMed
-
- Buechler C, Bauer S. ATP binding cassette transporter A1 (ABCA1) associated proteins: potential drug targets in the metabolic syndrome and atherosclerotic disease? Current pharmaceutical biotechnology. 2012;13:319–330. - PubMed
-
- Chung S, Timmins JM, Duong M, Degirolamo C, Rong S, Sawyer JK, Singaraja RR, Hayden MR, Maeda N, Rudel LL, Shelness GS, Parks JS. Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J Biol Chem. 2010;285:12197–12209. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

