Sinomenine hydrochloride protects against polymicrobial sepsis via autophagy
- PMID: 25625512
- PMCID: PMC4346851
- DOI: 10.3390/ijms16022559
Sinomenine hydrochloride protects against polymicrobial sepsis via autophagy
Abstract
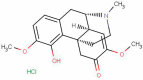
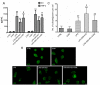
Sepsis, a systemic inflammatory response to infection, is the major cause of death in intensive care units (ICUs). The mortality rate of sepsis remains high even though the treatment and understanding of sepsis both continue to improve. Sinomenine (SIN) is a natural alkaloid extracted from Chinese medicinal plant Sinomenium acutum, and its hydrochloride salt (Sinomenine hydrochloride, SIN-HCl) is widely used to treat rheumatoid arthritis (RA). However, its role in sepsis remains unclear. In the present study, we investigated the role of SIN-HCl in sepsis induced by cecal ligation and puncture (CLP) in BALB/c mice and the corresponding mechanism. SIN-HCl treatment improved the survival of BALB/c mice that were subjected to CLP and reduced multiple organ dysfunction and the release of systemic inflammatory mediators. Autophagy activities were examined using Western blotting. The results showed that CLP-induced autophagy was elevated, and SIN-HCl treatment further strengthened the autophagy activity. Autophagy blocker 3-methyladenine (3-MA) was used to investigate the mechanism of SIN-HCl in vitro. Autophagy activities were determined by examining the autophagosome formation, which was shown as microtubule-associated protein light chain 3 (LC3) puncta with green immunofluorescence. SIN-HCl reduced lipopolysaccharide (LPS)-induced inflammatory cytokine release and increased autophagy in peritoneal macrophages (PM). 3-MA significantly decreased autophagosome formation induced by LPS and SIN-HCl. The decrease of inflammatory cytokines caused by SIN-HCl was partially aggravated by 3-MA treatment. Taken together, our results indicated that SIN-HCl could improve survival, reduce organ damage, and attenuate the release of inflammatory cytokines induced by CLP, at least in part through regulating autophagy activities.
Figures

Similar articles
-
Sinomenine attenuates septic-associated lung injury through the Nrf2-Keap1 and autophagy.J Pharm Pharmacol. 2020 Feb;72(2):259-270. doi: 10.1111/jphp.13202. Epub 2019 Nov 15. J Pharm Pharmacol. 2020. PMID: 31729764
-
Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets.Front Immunol. 2018 Sep 26;9:2228. doi: 10.3389/fimmu.2018.02228. eCollection 2018. Front Immunol. 2018. PMID: 30319663 Free PMC article. Clinical Trial.
-
Sinomenine ameliorates septic acute lung injury in mice by modulating gut homeostasis via aryl hydrocarbon receptor/Nrf2 pathway.Eur J Pharmacol. 2021 Dec 5;912:174581. doi: 10.1016/j.ejphar.2021.174581. Epub 2021 Oct 30. Eur J Pharmacol. 2021. PMID: 34743979
-
Cecal ligation and puncture-induced sepsis as a model to study autophagy in mice.J Vis Exp. 2014 Feb 9;(84):e51066. doi: 10.3791/51066. J Vis Exp. 2014. PMID: 24561344 Free PMC article. Review.
-
Recent Advances in the Therapeutic Potential of Sinomenine for Cancer Treatment.Pharmacology. 2024;109(2):76-85. doi: 10.1159/000536133. Epub 2024 Jan 30. Pharmacology. 2024. PMID: 38290489 Review.
Cited by
-
Sinomenine regulates immune cell subsets: Potential neuro-immune intervene for precise treatment of chronic pain.Front Cell Dev Biol. 2022 Dec 22;10:1041006. doi: 10.3389/fcell.2022.1041006. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36619869 Free PMC article. Review.
-
Intra-Articular Drug Delivery for Osteoarthritis Treatment.Pharmaceutics. 2021 Dec 15;13(12):2166. doi: 10.3390/pharmaceutics13122166. Pharmaceutics. 2021. PMID: 34959445 Free PMC article. Review.
-
Anti-inflammation Effects of Sinomenine on Macrophages through Suppressing Activated TLR4/NF-κB Signaling Pathway.Curr Med Sci. 2020 Feb;40(1):130-137. doi: 10.1007/s11596-020-2156-6. Epub 2020 Mar 13. Curr Med Sci. 2020. PMID: 32166675
-
Cell death signaling and immune regulation: new perspectives on targeted therapy for sepsis.Cell Mol Biol Lett. 2025 Aug 15;30(1):99. doi: 10.1186/s11658-025-00784-w. Cell Mol Biol Lett. 2025. PMID: 40817040 Free PMC article. Review.
-
The Yin and Yang dualistic features of autophagy in thermal burn wound healing.Int J Immunopathol Pharmacol. 2022 Jan-Dec;36:3946320221125090. doi: 10.1177/03946320221125090. Int J Immunopathol Pharmacol. 2022. PMID: 36121435 Free PMC article.
References
-
- Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348:138–150. - PubMed
-
- Riedemann N.C., Guo R.F., Ward P.A. Novel strategies for the treatment of sepsis. Nat. Med. 2003;9:517–524. - PubMed
-
- Levine B., Klionsky D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. - PubMed
-
- Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

