Spinal interneurons and forelimb plasticity after incomplete cervical spinal cord injury in adult rats
- PMID: 25625912
- PMCID: PMC4492610
- DOI: 10.1089/neu.2014.3718
Spinal interneurons and forelimb plasticity after incomplete cervical spinal cord injury in adult rats
Abstract
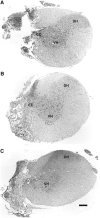
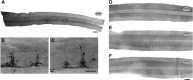
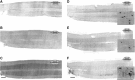
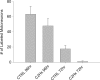
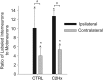
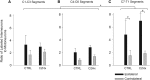
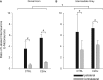
Cervical spinal cord injury (cSCI) disrupts bulbospinal projections to motoneurons controlling the upper limbs, resulting in significant functional impairments. Ongoing clinical and experimental research has revealed several lines of evidence for functional neuroplasticity and recovery of upper extremity function after SCI. The underlying neural substrates, however, have not been thoroughly characterized. The goals of the present study were to map the intraspinal motor circuitry associated with a defined upper extremity muscle, and evaluate chronic changes in the distribution of this circuit following incomplete cSCI. Injured animals received a high cervical (C2) lateral hemisection (Hx), which compromises supraspinal input to ipsilateral spinal motoneurons controlling the upper extremities (forelimb) in the adult rat. A battery of behavioral tests was used to characterize the time course and extent of forelimb motor recovery over a 16 week period post-injury. A retrograde transneuronal tracer - pseudorabies virus - was used to define the motor and pre-motor circuitry controlling the extensor carpi radialis longus (ECRL) muscle in spinal intact and injured animals. In the spinal intact rat, labeling was observed unilaterally within the ECRL motoneuron pool and within spinal interneurons bilaterally distributed within the dorsal horn and intermediate gray matter. No changes in labeling were observed 16 weeks post-injury, despite a moderate degree of recovery of forelimb motor function. These results suggest that recovery of the forelimb function assessed following C2Hx injury does not involve recruitment of new interneurons into the ipsilateral ECRL motor pathway. However, the functional significance of these existing interneurons to motor recovery requires further exploration.
Keywords: SCI; functional plasticity; propriospinal interneurons; upper extremity.
Figures




References
-
- Vizzard M., Erickson V., Card J., Roppolo J., and de Groat W. (1995). Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J. Comp. Neurol. 355, 629–640 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

