Natural variation in preparation for nutrient depletion reveals a cost-benefit tradeoff
- PMID: 25626068
- PMCID: PMC4308108
- DOI: 10.1371/journal.pbio.1002041
Natural variation in preparation for nutrient depletion reveals a cost-benefit tradeoff
Abstract
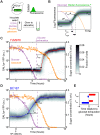
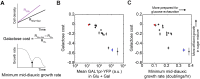
Maximizing growth and survival in the face of a complex, time-varying environment is a common problem for single-celled organisms in the wild. When offered two different sugars as carbon sources, microorganisms first consume the preferred sugar, then undergo a transient growth delay, the "diauxic lag," while inducing genes to metabolize the less preferred sugar. This delay is commonly assumed to be an inevitable consequence of selection to maximize use of the preferred sugar. Contrary to this view, we found that many natural isolates of Saccharomyces cerevisiae display short or nonexistent diauxic lags when grown in mixtures of glucose (preferred) and galactose. These strains induce galactose utilization (GAL) genes hours before glucose exhaustion, thereby "preparing" for the transition from glucose to galactose metabolism. The extent of preparation varies across strains, and seems to be determined by the steady-state response of GAL genes to mixtures of glucose and galactose rather than by induction kinetics. Although early GAL gene induction gives strains a competitive advantage once glucose runs out, it comes at a cost while glucose is still present. Costs and benefits correlate with the degree of preparation: strains with higher expression of GAL genes prior to glucose exhaustion experience a larger upfront growth cost but also a shorter diauxic lag. Our results show that classical diauxic growth is only one extreme on a continuum of growth strategies constrained by a cost-benefit tradeoff. This type of continuum is likely to be common in nature, as similar tradeoffs can arise whenever cells evolve to use mixtures of nutrients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

