Microfluidic devices for nucleic acid (NA) isolation, isothermal NA amplification, and real-time detection
- PMID: 25626529
- PMCID: PMC6540113
- DOI: 10.1007/978-1-4939-2172-0_2
Microfluidic devices for nucleic acid (NA) isolation, isothermal NA amplification, and real-time detection
Abstract
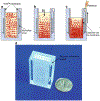
Molecular (nucleic acid)-based diagnostics tests have many advantages over immunoassays, particularly with regard to sensitivity and specificity. Most on-site diagnostic tests, however, are immunoassay-based because conventional nucleic acid-based tests (NATs) require extensive sample processing, trained operators, and specialized equipment. To make NATs more convenient, especially for point-of-care diagnostics and on-site testing, a simple plastic microfluidic cassette ("chip") has been developed for nucleic acid-based testing of blood, other clinical specimens, food, water, and environmental samples. The chip combines nucleic acid isolation by solid-phase extraction; isothermal enzymatic amplification such as LAMP (Loop-mediated AMPlification), NASBA (Nucleic Acid Sequence Based Amplification), and RPA (Recombinase Polymerase Amplification); and real-time optical detection of DNA or RNA analytes. The microfluidic cassette incorporates an embedded nucleic acid binding membrane in the amplification reaction chamber. Target nucleic acids extracted from a lysate are captured on the membrane and amplified at a constant incubation temperature. The amplification product, labeled with a fluorophore reporter, is excited with a LED light source and monitored in situ in real time with a photodiode or a CCD detector (such as available in a smartphone). For blood analysis, a companion filtration device that separates plasma from whole blood to provide cell-free samples for virus and bacterial lysis and nucleic acid testing in the microfluidic chip has also been developed. For HIV virus detection in blood, the microfluidic NAT chip achieves a sensitivity and specificity that are nearly comparable to conventional benchtop protocols using spin columns and thermal cyclers.
Figures
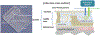




Similar articles
-
Simple Approaches to Minimally-Instrumented, Microfluidic-Based Point-of-Care Nucleic Acid Amplification Tests.Biosensors (Basel). 2018 Feb 26;8(1):17. doi: 10.3390/bios8010017. Biosensors (Basel). 2018. PMID: 29495424 Free PMC article. Review.
-
NAIL: Nucleic Acid detection using Isotachophoresis and Loop-mediated isothermal amplification.Lab Chip. 2015 Apr 7;15(7):1697-707. doi: 10.1039/c4lc01479k. Lab Chip. 2015. PMID: 25666345
-
Integrated Microfluidic Nucleic Acid Isolation, Isothermal Amplification, and Amplicon Quantification.Microarrays (Basel). 2015 Oct 20;4(4):474-89. doi: 10.3390/microarrays4040474. Microarrays (Basel). 2015. PMID: 27600235 Free PMC article. Review.
-
Integration of isothermal amplification methods in microfluidic devices: Recent advances.Biosens Bioelectron. 2017 Apr 15;90:174-186. doi: 10.1016/j.bios.2016.11.045. Epub 2016 Nov 19. Biosens Bioelectron. 2017. PMID: 27888686 Review.
-
Integrated sample-to-detection chip for nucleic acid test assays.Biomed Microdevices. 2016 Jun;18(3):44. doi: 10.1007/s10544-016-0069-8. Biomed Microdevices. 2016. PMID: 27165104
Cited by
-
"The Smartphone's Guide to the Galaxy": In Situ Analysis in Space.Biosensors (Basel). 2018 Oct 19;8(4):96. doi: 10.3390/bios8040096. Biosensors (Basel). 2018. PMID: 30347742 Free PMC article. Review.
-
Developing a surface acoustic wave-induced microfluidic cell lysis device for point-of-care DNA amplification.Eng Life Sci. 2023 Dec 6;24(1):e2300230. doi: 10.1002/elsc.202300230. eCollection 2024 Jan. Eng Life Sci. 2023. PMID: 38187928 Free PMC article.
-
Advances in Metagenomics and Its Application in Environmental Microorganisms.Front Microbiol. 2021 Dec 17;12:766364. doi: 10.3389/fmicb.2021.766364. eCollection 2021. Front Microbiol. 2021. PMID: 34975791 Free PMC article. Review.
-
Microfluidics-Based Nanobiosensors for Healthcare Monitoring.Mol Biotechnol. 2024 Mar;66(3):378-401. doi: 10.1007/s12033-023-00760-9. Epub 2023 May 11. Mol Biotechnol. 2024. PMID: 37166577 Free PMC article. Review.
-
Clinical evaluation of the loop-mediated isothermal amplification assay for the detection of common lower respiratory pathogens in patients with respiratory symptoms.Medicine (Baltimore). 2018 Dec;97(51):e13660. doi: 10.1097/MD.0000000000013660. Medicine (Baltimore). 2018. PMID: 30572483 Free PMC article.
References
-
- Chin CD, Linder V, Sia SK (2012) Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 12:2118–2134 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

