A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors
- PMID: 25626906
- PMCID: PMC4324313
- DOI: 10.1128/mBio.02456-14
A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors
Abstract
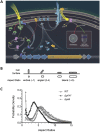
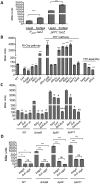
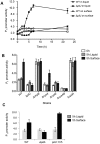
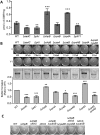
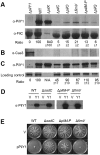
Biofilms are surface-attached multicellular communities. Using single-cell tracking microscopy, we showed that a pilY1 mutant of Pseudomonas aeruginosa is defective in early biofilm formation. We leveraged the observation that PilY1 protein levels increase on a surface to perform a genetic screen to identify mutants altered in surface-grown expression of this protein. Based on our genetic studies, we found that soon after initiating surface growth, cyclic AMP (cAMP) levels increase, dependent on PilJ, a chemoreceptor-like protein of the Pil-Chp complex, and the type IV pilus (TFP). cAMP and its receptor protein Vfr, together with the FimS-AlgR two-component system (TCS), upregulate the expression of PilY1 upon surface growth. FimS and PilJ interact, suggesting a mechanism by which Pil-Chp can regulate FimS function. The subsequent secretion of PilY1 is dependent on the TFP assembly system; thus, PilY1 is not deployed until the pilus is assembled, allowing an ordered signaling cascade. Cell surface-associated PilY1 in turn signals through the TFP alignment complex PilMNOP and the diguanylate cyclase SadC to activate downstream cyclic di-GMP (c-di-GMP) production, thereby repressing swarming motility. Overall, our data support a model whereby P. aeruginosa senses the surface through the Pil-Chp chemotaxis-like complex, TFP, and PilY1 to regulate cAMP and c-di-GMP production, thereby employing a hierarchical regulatory cascade of second messengers to coordinate its program of surface behaviors.
Importance: Biofilms are surface-attached multicellular communities. Here, we show that a stepwise regulatory circuit, involving ordered signaling via two different second messengers, is required for Pseudomonas aeruginosa to control early events in cell-surface interactions. We propose that our studies have uncovered a multilayered "surface-sensing" system that allows P. aeruginosa to effectively coordinate its surface-associated behaviors. Understanding how cells transition into the biofilm state on a surface may provide new approaches to prevent formation of these communities.
Copyright © 2015 Luo et al.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
