MiR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/β-catenin pathway
- PMID: 25627001
- PMCID: PMC4310092
- DOI: 10.1038/srep08087
MiR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/β-catenin pathway
Erratum in
-
Corrigendum: MiR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/β-catenin pathway.Sci Rep. 2017 Aug 24;7:46886. doi: 10.1038/srep46886. eCollection 2017. Sci Rep. 2017. PMID: 31305788 Free PMC article.
Abstract
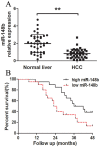
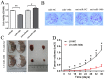
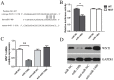
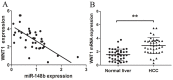
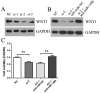
Accumulating evidences indicate that microRNAs play a vital role in regulating tumor progression. However, the roles of miR-148b in hepatocellular carcinoma (HCC) are still largely unknown. In this study, our data showed that miR-148b was significantly downregulated in 40 pairs of human HCC tissues. Further, the deregulated miR-148b was significantly correlated with larger tumor size, more tumor number, metastasis and worse prognosis in HCC. Overexpression of miR-148b inhibited HCC HepG2 cells proliferation and tumorigenicity. Further, miR-148b induced cells apoptosis by activating caspase- 3 and caspase-9, and induced S phase arrest by regulating cyclinD1 and p21, and also inhibited cell invasion. Data from the dual-luciferase reporter gene assay showed that WNT1 was a direct target of miR-148b, and overexpressed WNT1 inversely correlated with miR-148b levels in HCC tissues. Silencing of WNT1 inhibited the growth of HCC cells, and also induced cells apoptosis and inhibited invasion, which is consistent with the effects of miR-148b overexpression. MiR-148b downregulated expression of WNT1, β-catenin and C-myc, while upregulated E-cadherin expression. We conclude that the frequently downregulated miR-148b can regulate WNT1/β-catenin signalling pathway and function as a tumor suppressor in HCC. These findings suggest that miR-148b may serve as a novel therapeutic target for HCC.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

