Unbalanced activation of glutathione metabolic pathways suggests potential involvement in plant defense against the gall midge Mayetiola destructor in wheat
- PMID: 25627558
- PMCID: PMC4308708
- DOI: 10.1038/srep08092
Unbalanced activation of glutathione metabolic pathways suggests potential involvement in plant defense against the gall midge Mayetiola destructor in wheat
Abstract
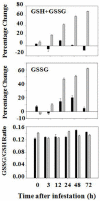
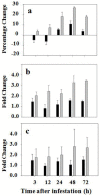
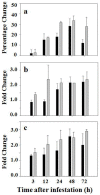
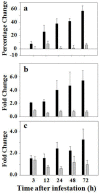
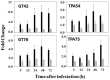
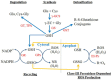
Glutathione, γ-glutamylcysteinylglycine, exists abundantly in nearly all organisms. Glutathione participates in various physiological processes involved in redox reactions by serving as an electron donor/acceptor. We found that the abundance of total glutathione increased up to 60% in resistant wheat plants within 72 hours following attack by the gall midge Mayetiola destructor, the Hessian fly. The increase in total glutathione abundance, however, is coupled with an unbalanced activation of glutathione metabolic pathways. The activity and transcript abundance of glutathione peroxidases, which convert reduced glutathione (GSH) to oxidized glutathione (GSSG), increased in infested resistant plants. However, the enzymatic activity and transcript abundance of glutathione reductases, which convert GSSG back to GSH, did not change. This unbalanced regulation of the glutathione oxidation/reduction cycle indicates the existence of an alternative pathway to regenerate GSH from GSSG to maintain a stable GSSG/GSH ratio. Our data suggest the possibility that GSSG is transported from cytosol to apoplast to serve as an oxidant for class III peroxidases to generate reactive oxygen species for plant defense against Hessian fly larvae. Our results provide a foundation for elucidating the molecular processes involved in glutathione-mediated plant resistance to Hessian fly and potentially other pests as well.
Figures
Similar articles
-
Hessian fly larval feeding triggers enhanced polyamine levels in susceptible but not resistant wheat.BMC Plant Biol. 2015 Jan 16;15:3. doi: 10.1186/s12870-014-0396-y. BMC Plant Biol. 2015. PMID: 25592131 Free PMC article.
-
Wheat Mds-1 encodes a heat-shock protein and governs susceptibility towards the Hessian fly gall midge.Nat Commun. 2013;4:2070. doi: 10.1038/ncomms3070. Nat Commun. 2013. PMID: 23792912
-
Reactive oxygen species are involved in plant defense against a gall midge.Plant Physiol. 2010 Feb;152(2):985-99. doi: 10.1104/pp.109.150656. Epub 2009 Dec 4. Plant Physiol. 2010. PMID: 19965963 Free PMC article.
-
Gall midges (Hessian flies) as plant pathogens.Annu Rev Phytopathol. 2012;50:339-57. doi: 10.1146/annurev-phyto-072910-095255. Epub 2012 May 29. Annu Rev Phytopathol. 2012. PMID: 22656645 Review.
-
Glutathione--linking cell proliferation to oxidative stress.Free Radic Biol Med. 2015 Dec;89:1154-64. doi: 10.1016/j.freeradbiomed.2015.09.023. Epub 2015 Nov 3. Free Radic Biol Med. 2015. PMID: 26546102 Review.
Cited by
-
Glutathione-Mediated Conjugation of Anticancer Drugs: An Overview of Reaction Mechanisms and Biological Significance for Drug Detoxification and Bioactivation.Molecules. 2022 Aug 17;27(16):5252. doi: 10.3390/molecules27165252. Molecules. 2022. PMID: 36014491 Free PMC article. Review.
-
Metabolomic Profiling Identifies Key Metabolites and Defense Pathways in Rlm1-Mediated Blackleg Resistance in Canola.Int J Mol Sci. 2025 Jun 12;26(12):5627. doi: 10.3390/ijms26125627. Int J Mol Sci. 2025. PMID: 40565092 Free PMC article.
-
Seasonal phoresy as an overwintering strategy of a phytophagous mite.Sci Rep. 2016 May 6;6:25483. doi: 10.1038/srep25483. Sci Rep. 2016. PMID: 27150196 Free PMC article.
-
Expression Patterns of Genes Involved in Ascorbate-Glutathione Cycle in Aphid-Infested Maize (Zea mays L.) Seedlings.Int J Mol Sci. 2016 Feb 23;17(3):268. doi: 10.3390/ijms17030268. Int J Mol Sci. 2016. PMID: 26907270 Free PMC article.
-
Impact of Heat Stress on Expression of Wheat Genes Responsive to Hessian Fly Infestation.Plants (Basel). 2022 May 25;11(11):1402. doi: 10.3390/plants11111402. Plants (Basel). 2022. PMID: 35684175 Free PMC article.
References
-
- Noctor G. et al. Glutathione in plants: an integrated overview. Plant, Cell Environ. 35, 454–484 (2012). - PubMed
-
- Noctor G. et al. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot. 49, 623–647 (1998).
-
- Marrs K. A. The functions and regulation of glutathione S-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 127–158 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

