Spatio-Temporal Control of LbL Films for Biomedical Applications: From 2D to 3D
- PMID: 25627563
- PMCID: PMC4540079
- DOI: 10.1002/adhm.201400715
Spatio-Temporal Control of LbL Films for Biomedical Applications: From 2D to 3D
Abstract
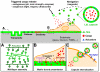
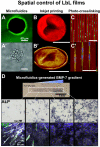
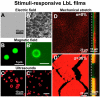
Introduced in the '90s by Prof. Moehwald, Lvov, and Decher, the layer-by-layer (LbL) assembly of polyelectrolytes has become a popular technique to engineer various types of objects such as films, capsules and free standing membranes, with an unprecedented control at the nanometer and micrometer scales. The LbL technique allows to engineer biofunctional surface coatings, which may be dedicated to biomedical applications in vivo but also to fundamental studies and diagnosis in vitro. Initially mostly developed as 2D coatings and hollow capsules, the range of complex objects created by the LbL technique has greatly expanded in the past 10 years. In this Review, the aim is to highlight the recent progress in the field of LbL films for biomedical applications and to discuss the various ways to spatially and temporally control the biochemical and mechanical properties of multilayers. In particular, three major developments of LbL films are discussed: 1) the new methods and templates to engineer LbL films and control cellular processes from adhesion to differentiation, 2) the major ways to achieve temporal control by chemical, biological and physical triggers and, 3) the combinations of LbL technique, cells and scaffolds for repairing 3D tissues, including cardio-vascular devices, bone implants and neuro-prosthetic devices.
Keywords: drug delivery; layer-by-layer; scaffolds; spatial; temporal; tissue engineering.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Farhatnia Y, Tan A, Motiwala A, Cousins BG, Seifalian AM. Biotechnol. Adv. 2013;31:524. - PubMed
-
- Decher G. Science. 1997;277:1232. (80-. )
-
- Moya S, Sukhorukov G, Auch M, Donath E, Möhwald H. J. Colloid Interface Sci. 1999;216:297. - PubMed
-
- Lvov Y, Onda M, Ariga K, Kunitake T. J. Biomater. Sci. Polym. Ed. 1998;9:345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

