Myofibroblasts exhibit enhanced fibronectin assembly that is intrinsic to their contractile phenotype
- PMID: 25627685
- PMCID: PMC4358119
- DOI: 10.1074/jbc.M114.606186
Myofibroblasts exhibit enhanced fibronectin assembly that is intrinsic to their contractile phenotype
Abstract
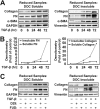
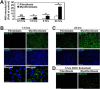
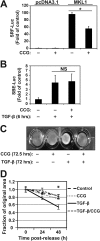
Myofibroblasts have increased expression of contractile proteins and display augmented contractility. It is not known if the augmented contractile gene expression characterizing the myofibroblast phenotype impacts its intrinsic ability to assemble fibronectin (FN) and extracellular matrix. In this study we investigated whether myofibroblasts displayed increased rates of FN fibril assembly when compared with their undifferentiated counterparts. Freshly plated myofibroblasts assemble exogenous FN (488-FN) into a fibrillar matrix more rapidly than fibroblasts that have not undergone myofibroblast differentiation. The augmented rate of FN matrix formation by myofibroblasts was dependent on intact Rho/Rho kinase (ROCK) and myosin signals inasmuch as treatment with Y27632 or blebbistatin attenuated 488-FN assembly. Inhibiting contractile gene expression by pharmacologic disruption of the transcription factors megakaryoblastic leukemia-1 (MKL1)/serum response factor (SRF) during myofibroblast differentiation resulted in decreased contractile force generation and attenuated 488-FN incorporation although not FN expression. Furthermore, disruption of the MKL1/SRF target gene, smooth muscle α-actin (α-SMA) via siRNA knockdown resulted in attenuation of 488-FN assembly. In conclusion, this study demonstrates a linkage between increased contractile gene expression, most importantly α-SMA, and the intrinsic capacity of myofibroblasts to assemble exogenous FN into fibrillar extracellular matrix.
Keywords: Extracellular Matrix; Fibroblast; Fibronectin; Megakaryoblastic Leukemia-1; Myofibroblast; Serum Response Factor; Smooth Muscle α-Actin; Transforming Growth Factor β (TGF-B).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Tensin 1 Is Essential for Myofibroblast Differentiation and Extracellular Matrix Formation.Am J Respir Cell Mol Biol. 2017 Apr;56(4):465-476. doi: 10.1165/rcmb.2016-0104OC. Am J Respir Cell Mol Biol. 2017. PMID: 28005397 Free PMC article.
-
Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-β.Am J Physiol Lung Cell Mol Physiol. 2011 Nov;301(5):L656-66. doi: 10.1152/ajplung.00166.2011. Epub 2011 Aug 19. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21856814 Free PMC article.
-
Control of myofibroblast differentiation by microtubule dynamics through a regulated localization of mDia2.J Biol Chem. 2013 May 31;288(22):15466-73. doi: 10.1074/jbc.M113.464461. Epub 2013 Apr 11. J Biol Chem. 2013. PMID: 23580645 Free PMC article.
-
The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation.J Cardiovasc Transl Res. 2012 Dec;5(6):794-804. doi: 10.1007/s12265-012-9397-0. Epub 2012 Aug 17. J Cardiovasc Transl Res. 2012. PMID: 22898751 Review.
-
Myofibroblasts: trust your heart and let fate decide.J Mol Cell Cardiol. 2014 May;70:9-18. doi: 10.1016/j.yjmcc.2013.10.019. Epub 2013 Nov 2. J Mol Cell Cardiol. 2014. PMID: 24189039 Free PMC article. Review.
Cited by
-
Extracellular matrix remodeling associated with bleomycin-induced lung injury supports pericyte-to-myofibroblast transition.Matrix Biol Plus. 2020 Dec 30;10:100056. doi: 10.1016/j.mbplus.2020.100056. eCollection 2021 Jun. Matrix Biol Plus. 2020. PMID: 34195593 Free PMC article.
-
Megakaryoblastic leukemia-1 is required for the development of bleomycin-induced pulmonary fibrosis.Respir Res. 2015 Mar 27;16(1):45. doi: 10.1186/s12931-015-0206-6. Respir Res. 2015. PMID: 25885656 Free PMC article.
-
HDAC6 Regulates the MRTF-A/SRF Axis and Vascular Smooth Muscle Cell Plasticity.JACC Basic Transl Sci. 2018 Dec 31;3(6):782-795. doi: 10.1016/j.jacbts.2018.08.010. eCollection 2018 Dec. JACC Basic Transl Sci. 2018. PMID: 30623138 Free PMC article.
-
BMPR2 acts as a gatekeeper to protect endothelial cells from increased TGFβ responses and altered cell mechanics.PLoS Biol. 2019 Dec 11;17(12):e3000557. doi: 10.1371/journal.pbio.3000557. eCollection 2019 Dec. PLoS Biol. 2019. PMID: 31826007 Free PMC article.
-
Spatiotemporal control of myofibroblast activation in acoustically-responsive scaffolds via ultrasound-induced matrix stiffening.Acta Biomater. 2022 Jan 15;138:133-143. doi: 10.1016/j.actbio.2021.11.020. Epub 2021 Nov 20. Acta Biomater. 2022. PMID: 34808418 Free PMC article.
References
-
- Phan S. H. (1996) Role of the myofibroblast in pulmonary fibrosis. Kidney Int. Suppl. 54, S46–S48 - PubMed
-
- Gabbiani G., Ryan G. B., Majne G. (1971) Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27, 549–550 - PubMed
-
- Malmström J., Lindberg H., Lindberg C., Bratt C., Wieslander E., Delander E. L., Särnstrand B., Burns J. S., Mose-Larsen P., Fey S., Marko-Varga G. (2004) Transforming growth factor-β1 specifically induce proteins involved in the myofibroblast contractile apparatus. Mol. Cell. Proteomics 3, 466–477 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

