The acquisition of novel N-glycosylation sites in conserved proteins during human evolution
- PMID: 25628020
- PMCID: PMC4314935
- DOI: 10.1186/s12859-015-0468-5
The acquisition of novel N-glycosylation sites in conserved proteins during human evolution
Abstract
Background: N-linked protein glycosylation plays an important role in various biological processes, including protein folding and trafficking, and cell adhesion and signaling. The acquisition of a novel N-glycosylation site may have significant effect on protein structure and function, and therefore, on the phenotype.
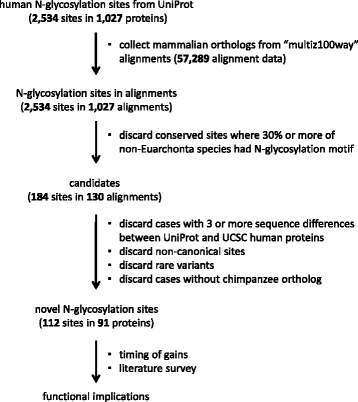
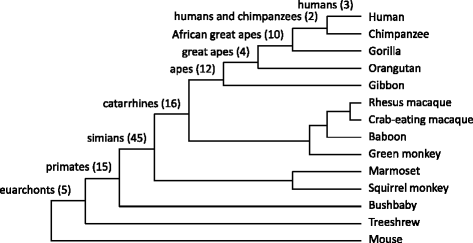
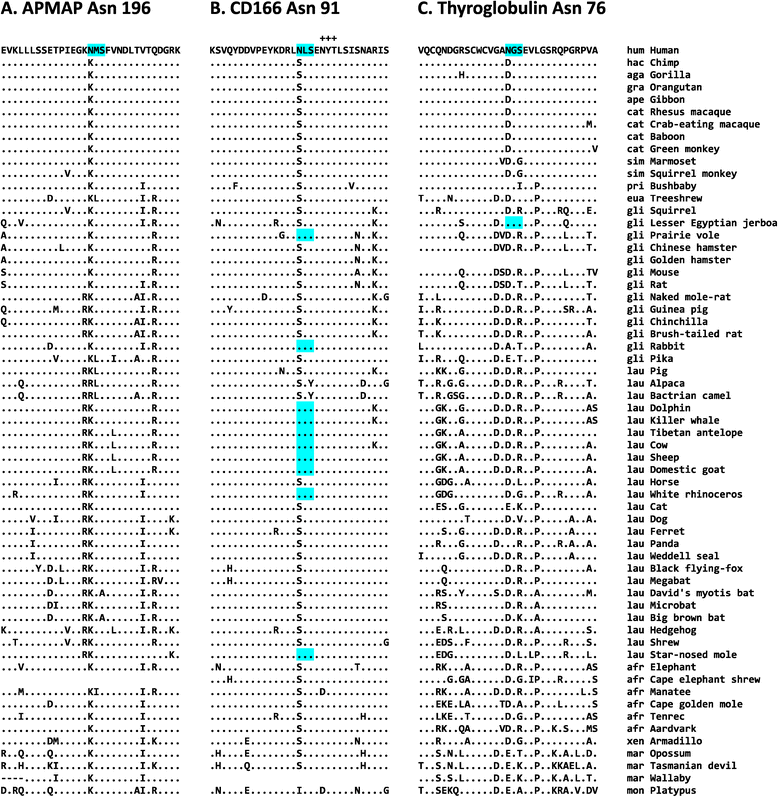
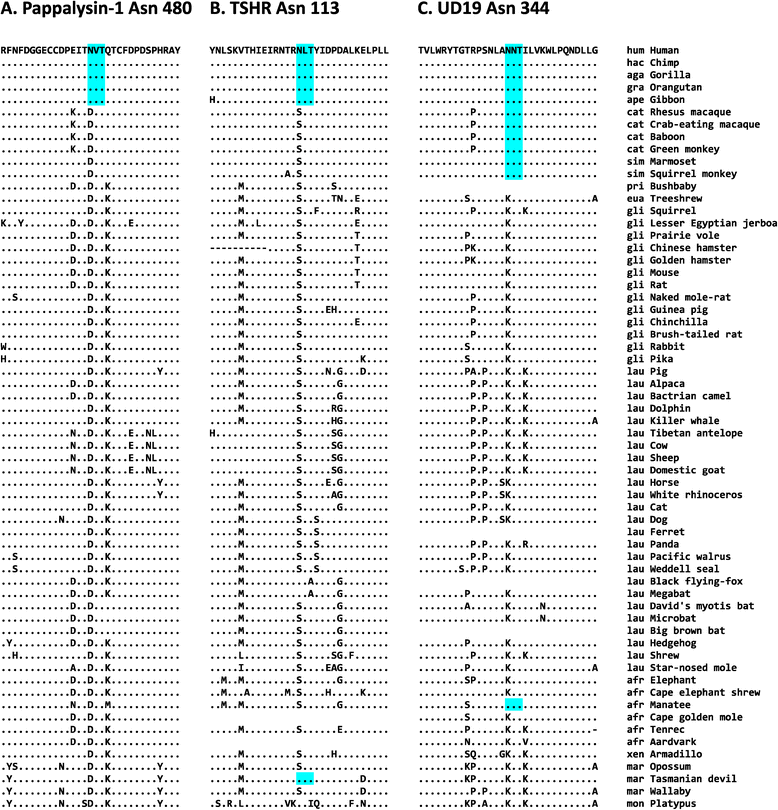
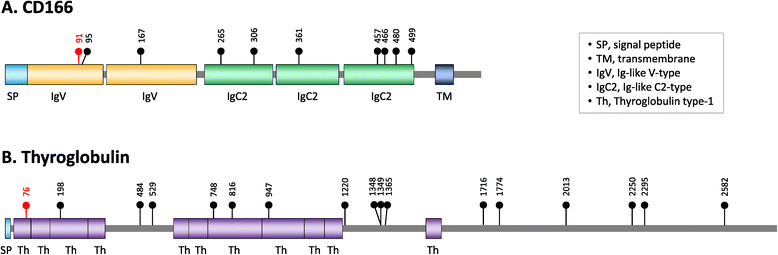
Results: We analyzed the human glycoproteome data set (2,534 N-glycosylation sites in 1,027 proteins) and identified 112 novel N-glycosylation sites in 91 proteins that arose in the human lineage since the last common ancestor of Euarchonta (primates and treeshrews). Three of them, Asn-196 in adipocyte plasma membrane-associated protein (APMAP), Asn-91 in cluster of differentiation 166 (CD166/ALCAM), and Asn-76 in thyroglobulin, are human-specific. Molecular evolutionary analysis suggested that these sites were under positive selection during human evolution. Notably, the Asn-76 of thyroglobulin might be involved in the increased production of thyroid hormones in humans, especially thyroxine (T4), because the removal of the glycan moiety from this site was reported to result in a significant decrease in T4 production.
Conclusions: We propose that the novel N-glycosylation sites described in this study may be useful candidates for functional analyses to identify innovative genetic modifications for beneficial phenotypes acquired in the human lineage.
Figures
Similar articles
-
Loss of ancestral N-glycosylation sites in conserved proteins during human evolution.Int J Mol Med. 2015 Dec;36(6):1685-92. doi: 10.3892/ijmm.2015.2362. Epub 2015 Oct 7. Int J Mol Med. 2015. PMID: 26458842
-
Glycosylation-dependent binding of galectin-8 to activated leukocyte cell adhesion molecule (ALCAM/CD166) promotes its surface segregation on breast cancer cells.Biochim Biophys Acta. 2016 Oct;1860(10):2255-68. doi: 10.1016/j.bbagen.2016.04.019. Epub 2016 Apr 26. Biochim Biophys Acta. 2016. PMID: 27130882
-
Glycosylation in human thyroglobulin: location of the N-linked oligosaccharide units and comparison with bovine thyroglobulin.Arch Biochem Biophys. 1996 Mar 1;327(1):61-70. doi: 10.1006/abbi.1996.0093. Arch Biochem Biophys. 1996. PMID: 8615697
-
Probing into the role of conserved N-glycosylation sites in the Tyrosinase glycoprotein family.Glycoconj J. 2009 Aug;26(6):691-5. doi: 10.1007/s10719-008-9213-x. Epub 2008 Nov 13. Glycoconj J. 2009. PMID: 19015978 Review.
-
Effects of N-glycan precursor length diversity on quality control of protein folding and on protein glycosylation.Semin Cell Dev Biol. 2015 May;41:121-8. doi: 10.1016/j.semcdb.2014.11.008. Epub 2014 Dec 2. Semin Cell Dev Biol. 2015. PMID: 25475176 Free PMC article. Review.
Cited by
-
Differential effects of N-linked glycosylation of Vstm5 at multiple sites on surface expression and filopodia formation.PLoS One. 2017 Jul 26;12(7):e0181257. doi: 10.1371/journal.pone.0181257. eCollection 2017. PLoS One. 2017. PMID: 28746350 Free PMC article.
-
N-GlycositeAtlas: a database resource for mass spectrometry-based human N-linked glycoprotein and glycosylation site mapping.Clin Proteomics. 2019 Sep 7;16:35. doi: 10.1186/s12014-019-9254-0. eCollection 2019. Clin Proteomics. 2019. PMID: 31516400 Free PMC article.
-
Charged amino acid variability related to N-glyco -sylation and epitopes in A/H3N2 influenza: Hem -agglutinin and neuraminidase.PLoS One. 2017 Jul 14;12(7):e0178231. doi: 10.1371/journal.pone.0178231. eCollection 2017. PLoS One. 2017. PMID: 28708860 Free PMC article.
-
Identification of Novel N-Glycosylation Sites at Noncanonical Protein Consensus Motifs.J Proteome Res. 2016 Jul 1;15(7):2087-101. doi: 10.1021/acs.jproteome.5b00733. Epub 2016 Jun 14. J Proteome Res. 2016. PMID: 27246700 Free PMC article.
-
In vitro toxicity model: Upgrades to bridge the gap between preclinical and clinical research.Bosn J Basic Med Sci. 2020 May 1;20(2):157-168. doi: 10.17305/bjbms.2019.4378. Bosn J Basic Med Sci. 2020. PMID: 31621554 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

