The p.Cys169Tyr variant of connexin 26 is not a polymorphism
- PMID: 25628337
- PMCID: PMC4383868
- DOI: 10.1093/hmg/ddv026
The p.Cys169Tyr variant of connexin 26 is not a polymorphism
Abstract
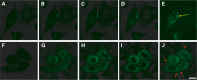
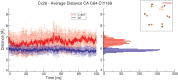
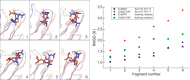
Mutations in the GJB2 gene, which encodes the gap junction protein connexin 26 (Cx26), are the primary cause of hereditary prelingual hearing impairment. Here, the p.Cys169Tyr missense mutation of Cx26 (Cx26C169Y), previously classified as a polymorphism, has been identified as causative of severe hearing loss in two Qatari families. We have analyzed the effect of this mutation using a combination of confocal immunofluorescence microscopy and molecular dynamics simulations. At the cellular level, our results show that the mutant protein fails to form junctional channels in HeLa transfectants despite being correctly targeted to the plasma membrane. At the molecular level, this effect can be accounted for by disruption of the disulfide bridge that Cys169 forms with Cys64 in the wild-type structure (Cx26WT). The lack of the disulfide bridge in the Cx26C169Y protein causes a spatial rearrangement of two important residues, Asn176 and Thr177. In the Cx26WT protein, these residues play a crucial role in the intra-molecular interactions that permit the formation of an intercellular channel by the head-to-head docking of two opposing hemichannels resident in the plasma membrane of adjacent cells. Our results elucidate the molecular pathogenesis of hereditary hearing loss due to the connexin mutation and facilitate the understanding of its role in both healthy and affected individuals.
© The Author 2015. Published by Oxford University Press.
Figures

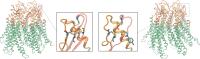
References
-
- Sohl G., Willecke K. (2003) An update on connexin genes and their nomenclature in mouse and man. Cell. Commun. Adhes., 10, 173–180. - PubMed
-
- Maeda S., Nakagawa S., Suga M., Yamashita E., Oshima A., Fujiyoshi Y., Tsukihara T. (2009) Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature, 458, 597–602. - PubMed
-
- Oshima A. (2014) Structure and closure of connexin gap junction channels. FEBS Lett., 588, 1230–1237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

