FutureTox II: in vitro data and in silico models for predictive toxicology
- PMID: 25628403
- PMCID: PMC4318934
- DOI: 10.1093/toxsci/kfu234
FutureTox II: in vitro data and in silico models for predictive toxicology
Abstract
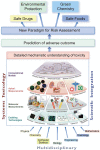
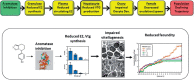
FutureTox II, a Society of Toxicology Contemporary Concepts in Toxicology workshop, was held in January, 2014. The meeting goals were to review and discuss the state of the science in toxicology in the context of implementing the NRC 21st century vision of predicting in vivo responses from in vitro and in silico data, and to define the goals for the future. Presentations and discussions were held on priority concerns such as predicting and modeling of metabolism, cell growth and differentiation, effects on sensitive subpopulations, and integrating data into risk assessment. Emerging trends in technologies such as stem cell-derived human cells, 3D organotypic culture models, mathematical modeling of cellular processes and morphogenesis, adverse outcome pathway development, and high-content imaging of in vivo systems were discussed. Although advances in moving towards an in vitro/in silico based risk assessment paradigm were apparent, knowledge gaps in these areas and limitations of technologies were identified. Specific recommendations were made for future directions and research needs in the areas of hepatotoxicity, cancer prediction, developmental toxicity, and regulatory toxicology.
Keywords: in silico; in vitro; modeling; predictive toxicology; risk assessment.
© The Author 2015. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Andersen M. E., Krewski D. (2010). The vision of toxicity testing in the 21st century: Moving from discussion to action. Toxicol. Sci. 117, 17–24. - PubMed
-
- Ankley G. T., Bennett R. S., Erickson R. J., Hoff D. J., Hornung M. W., Johnson R. D., Mount D. R., Nichols J. W., Russom C. L., Schmieder P. K., et al. (2010). Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29, 730–741. - PubMed
-
- Bus J. S., Becker R. A. (2009). Toxicity testing in the 21st century: A view from the chemical industry. Toxicol. Sci. 112, 297–302. - PubMed
-
- Chapin R. E., Stedman D. B. (2009). Endless possibilities: Stem cells and the vision for toxicology testing in the 21st century. Toxicol. Sci. 112, 17–22. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

