Phosphorylation of FADD by the kinase CK1α promotes KRASG12D-induced lung cancer
- PMID: 25628462
- PMCID: PMC4416214
- DOI: 10.1126/scisignal.2005607
Phosphorylation of FADD by the kinase CK1α promotes KRASG12D-induced lung cancer
Erratum in
- Sci Signal. 2015 Mar 17;8(368):er3
Abstract
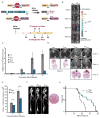
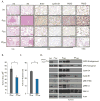
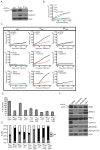
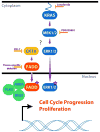
Genomic amplification of the gene encoding and phosphorylation of the protein FADD (Fas-associated death domain) is associated with poor clinical outcome in lung cancer and in head and neck cancer. Activating mutations in the guanosine triphosphatase RAS promotes cell proliferation in various cancers. Increased abundance of phosphorylated FADD in patient-derived tumor samples predicts poor clinical outcome. Using immunohistochemistry analysis and in vivo imaging of conditional mouse models of KRAS(G12D)-driven lung cancer, we found that the deletion of the gene encoding FADD suppressed tumor growth, reduced the proliferative index of cells, and decreased the activation of downstream effectors of the RAS-MAPK (mitogen-activated protein kinase) pathway that promote the cell cycle, including retinoblastoma (RB) and cyclin D1. In mouse embryonic fibroblasts, the induction of mitosis upon activation of KRAS required FADD and the phosphorylation of FADD by CK1α (casein kinase 1α). Deleting the gene encoding CK1α in KRAS mutant mice abrogated the phosphorylation of FADD and suppressed lung cancer development. Phosphorylated FADD was most abundant during the G2/M phase of the cell cycle, and mass spectrometry revealed that phosphorylated FADD interacted with kinases that mediate the G2/M transition, including PLK1 (Polo-like kinase 1), AURKA (Aurora kinase A), and BUB1 (budding uninhibited by benzimidazoles 1). This interaction was decreased in cells treated with a CKI-7, a CK1α inhibitor. Therefore, as the kinase that phosphorylates FADD downstream of RAS, CK1α may be a therapeutic target for KRAS-driven lung cancer.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures
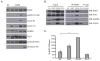

Similar articles
-
Phosphorylation by polo-like kinase 1 induces the tumor-suppressing activity of FADD.Oncogene. 2011 Jan 27;30(4):471-81. doi: 10.1038/onc.2010.423. Epub 2010 Oct 4. Oncogene. 2011. PMID: 20890306
-
Cooperative phosphorylation of FADD by Aur-A and Plk1 in response to taxol triggers both apoptotic and necrotic cell death.Cancer Res. 2011 Dec 1;71(23):7207-15. doi: 10.1158/0008-5472.CAN-11-0760. Epub 2011 Oct 6. Cancer Res. 2011. PMID: 21978935
-
Opioid receptor agonists enhance the phosphorylation state of Fas-associated death domain (FADD) protein in the rat brain: functional interactions with casein kinase Ialpha, Galpha(i) proteins, and ERK1/2 signaling.Neuropharmacology. 2008 Oct;55(5):886-99. doi: 10.1016/j.neuropharm.2008.06.071. Epub 2008 Jul 9. Neuropharmacology. 2008. PMID: 18657552
-
Nuclear localized phosphorylated FADD induces cell proliferation and is associated with aggressive lung cancer.Cell Cycle. 2005 Nov;4(11):1478-81. doi: 10.4161/cc.4.11.2188. Epub 2005 Nov 20. Cell Cycle. 2005. PMID: 16258269 Review.
-
Targeting Protein Kinases in Blood Cancer: Focusing on CK1α and CK2.Int J Mol Sci. 2021 Apr 2;22(7):3716. doi: 10.3390/ijms22073716. Int J Mol Sci. 2021. PMID: 33918307 Free PMC article. Review.
Cited by
-
Prognostic significance and immune correlates of FADD in penile squamous cell carcinoma.Virchows Arch. 2023 May;482(5):869-878. doi: 10.1007/s00428-023-03514-9. Epub 2023 Feb 23. Virchows Arch. 2023. PMID: 36813950
-
Heterogeneous genomic aberrations in esophageal squamous cell carcinoma: a review.Am J Transl Res. 2020 May 15;12(5):1553-1568. eCollection 2020. Am J Transl Res. 2020. PMID: 32509161 Free PMC article. Review.
-
Keeping autophagy in cheCK1.Mol Cell Oncol. 2015 May 26;3(3):e1045117. doi: 10.1080/23723556.2015.1045117. eCollection 2016 May. Mol Cell Oncol. 2015. PMID: 27314070 Free PMC article.
-
Thymoquinone Alterations of the Apoptotic Gene Expressions and Cell Cycle Arrest in Genetically Distinct Triple-Negative Breast Cancer Cells.Nutrients. 2022 May 19;14(10):2120. doi: 10.3390/nu14102120. Nutrients. 2022. PMID: 35631261 Free PMC article.
-
The renewed battle against RAS-mutant cancers.Cell Mol Life Sci. 2016 May;73(9):1845-58. doi: 10.1007/s00018-016-2155-8. Epub 2016 Feb 18. Cell Mol Life Sci. 2016. PMID: 26892781 Free PMC article. Review.
References
-
- Liu X, Yan S, Zhou T, Terada Y, Erikson RL. The MAP kinase pathway is required for entry into mitosis and cell survival. Oncogene. 2004;23:763–776. - PubMed
-
- Tourneur L, Chiocchia G. FADD: a regulator of life and death. Trends Immunol. 2010;31:260–269. - PubMed
-
- Gómez-Angelats M, Cidlowski JA. Molecular evidence for the nuclear localization of FADD. Cell Death Differ. 2003;10:791–797. - PubMed
-
- Chen G, Bhojani MS, Heaford AC, Chang DC, Laxman B, Thomas DG, Griffin LB, Yu J, Coppola JM, Giordano TJ, Lin L, Adams D, Orringer MB, Ross BD, Beer DG, Rehemtulla A. Phosphorylated FADD induces NF-κB, perturbs cell cycle, and is associated with poor outcome in lung adenocarcinomas. PNAS. 2005;102:12507–12512. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

