Transthyretin cardiac amyloidosis: pathogenesis, treatments, and emerging role in heart failure with preserved ejection fraction
- PMID: 25628512
- PMCID: PMC4284988
- DOI: 10.4137/CMC.S15719
Transthyretin cardiac amyloidosis: pathogenesis, treatments, and emerging role in heart failure with preserved ejection fraction
Abstract
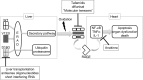
Transthyretin (TTR) amyloidosis causes heart failure from cardiac deposition of TTR amyloid fibrils, the by-product of TTR homotetramer disassembly. Wild-type (WT) TTR deposition leads to senile amyloidosis, predominantly manifesting with cardiomyopathy. Missense mutations in the TTR gene result in familial TTR amyloidosis. Certain mutations are more likely to affect the heart, while others cause more neurologic involvement. Extracellular fibril deposition triggers intracellular stress response, upregulation of the inflammatory cascades, apoptosis, and organ dysfunction. Recent studies suggest that TTR cardiac amyloid may be a significant contributor to the pathogenesis of heart failure with preserved ejection fraction (HFpEF). Summarized in this review are the molecular pathways underlying the cellular toxicity of TTR amyloid fibrils and the emerging therapies aimed at TTR tetramer stabilization, abrogation of TTR synthesis in the liver, or inhibition of amyloidogenesis.
Keywords: cardiac amyloidosis; heart failure with preserved ejection fraction (HFpEF); transthyretin (TTR).
Figures

References
-
- Dungu JN, Anderson LJ, Whelan CJ, Hawkins PN. Cardiac transthyretin amyloidosis. Heart. 2012;98(21):1546–54. - PubMed
-
- Coelho T, Maurer MS, Suhr OB. THAOS – the transthyretin amyloidosis outcomes survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63–76. - PubMed
-
- Yamashita T, Asl KH, Yazaki M, Benson MD. A prospective evaluation of the transthyretin Ile122 allele frequency in an African-American population. Amyloid. 2005;12(2):127–30. - PubMed
-
- Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336(7):466–73. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

