Anti-inflammatory effects of levalbuterol-induced 11β-hydroxysteroid dehydrogenase type 1 activity in airway epithelial cells
- PMID: 25628603
- PMCID: PMC4290686
- DOI: 10.3389/fendo.2014.00236
Anti-inflammatory effects of levalbuterol-induced 11β-hydroxysteroid dehydrogenase type 1 activity in airway epithelial cells
Abstract
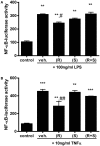
Airway epithelial NF-κB activation is observed in asthmatic subjects and is a cause of airway inflammation in mouse models of allergic asthma. Combination therapy with inhaled short-acting β2-agonists and corticosteroids significantly improves lung function and reduces inflammation in asthmatic subjects. Corticosteroids operate through a number of mechanisms to potently inhibit NF-κB activity. Since β2-agonists can induce expression of 11β-HSD1, which converts inactive 11-keto corticosteroids into active 11-hydroxy corticosteroids, thereby potentiating the effects of endogenous glucocorticoids, we examined whether this mechanism is involved in the inhibition of NF-κB activation induced by the β-agonist albuterol in airway epithelial cells. Treatment of transformed murine Club cells (MTCC) with (R)-albuterol (levalbuterol), but not with (S)- or a mixture of (R + S)- (racemic) albuterol, augmented mRNA expression of 11β-HSD1. MTCC were stably transfected with luciferase (luc) reporter constructs under transcriptional regulation by NF-κB (NF-κB/luc) or glucocorticoid response element (GRE/luc) consensus motifs. Stimulation of NF-κB/luc MTCC with lipopolysaccharide (LPS) or tumor necrosis factor-α (TNFα) induced luc activity, which was inhibited by pretreatment with (R)-, but not (S)- or racemic albuterol. Furthermore, pretreatment of GRE/luc MTCC with (R)-, but not with (S)- or racemic albuterol, augmented 11-keto corticosteroid (cortisone) induced luc activity, which was diminished by the 11β-HSD inhibitor glycyrrhetinic acid (18β-GA), indicating that there was a conversion of inactive 11-keto to active 11-hydroxy corticosteroids. LPS- and TNFα-induced NF-κB/luc activity was diminished in MTCC cells treated with a combination of cortisone and (R)-albuterol, an effect that was inhibited by 18β-GA. Finally, pretreatment of MTCC cells with the combination of cortisone and (R)-albuterol diminished LPS- and TNFα-induced pro-inflammatory cytokine production to an extent similar to that of dexamethasone. These results demonstrate that levalbuterol augments expression of 11β-HSD1 in airway epithelial cells, reducing LPS-induced NF-κB transcriptional activity and pro-inflammatory cytokine production through the conversion of inactive 11-keto corticosteroids into the active 11-hydroxy form in this cell type.
Keywords: 11beta-hydroxysteroid dehydrogenase; albuterol; anti-inflammatory; epithelium; glucocorticoid.
Figures




References
-
- Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief (2012) 94:1–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

