High-dose continuous-infusion ifosfamide in advanced well-differentiated/dedifferentiated liposarcoma
- PMID: 25628856
- PMCID: PMC4307996
- DOI: 10.1186/2045-3329-4-16
High-dose continuous-infusion ifosfamide in advanced well-differentiated/dedifferentiated liposarcoma
Abstract
Background: Liposarcomas represent the most common histological type of soft-tissue sarcomas (STS). Its main subgroups, WD/DD, is known to be poorly sensitive to chemotherapy, with few active agents, i.e., anthracyclines +/- ifosfamide and trabectedin. High-dose ifosfamide (HDIFX >12 g/m2) is active in STS pts pretreated with standard-dose IFX, though with greater toxicity. A prolonged continuous-infusion (ci) through a portable external pump may be an alternative way to administer HDIFX.
Methods: From March 2002 to August 2013, 28 pts (median age =60, range =37-73 yrs) with advanced disease (6 WD and 22 WD/DD) were given ciHDIFX, at the dose of 14 g/m2 as a 14-day continuous infusion every 4 weeks. Twenty-four pts (86%) were previously treated with chemotherapy (19 with anthracyclines and ifosfamide; 4 with anthracycline monotherapy; 1 with trabectedin).
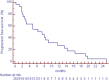
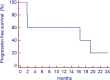
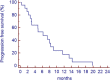
Results: Seven PR (all in DDLPS), 2 minor response (MR) and 11 SD were observed. Of interest, 6 of 9 patients with PR or MR had had SD with the previous therapy with anthracycline plus ifosfamide. The median progression-free survival was 7 months. Most common side effects were mild myelosuppression (anemia G2-3 in 3 pts; G2-3 neutropenia in 3 pts and G4 in 1; G3 thrombocytopenia in 1 pt); nausea (G3 in 3 pts) and fatigue (G3 in 6 pts). One pts had transient G3 confusion.
Conclusions: These data suggest that ciHDIFX is active in WD/DDLPS, even in patients already treated with a combination of anthracyclines plus ifosfamide. In this series, ciHDIFX regimen was better tolerated than HDIFX in published studies.
Keywords: Chemotherapy; Ifosfamide; Liposarcoma; Soft tissue sarcoma.
Figures
References
-
- Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO Classification of Tumours of Bone and Soft Tissue. Lyon: IARC; 2013. pp. 33–36.
-
- Dei Tos AP, Pedetour F. Well-differentiated liposarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO Classification of Soft Tissue Tumor and Bone. Lyon: IARC; 2013. pp. 33–36.
-
- Dei Tos AP, Marino-Enriquez A, Pedetour F, Rossi S. Dedifferentiated liposarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO Classification of Soft Tissue Tumor and Bone. IARC: Lyon; 2013. pp. 37–38.
-
- Binh MB, Sastre-Garau X, Guillou L, de Pinieux G, Terrier P, Lagacé R, Aurias A, Hostein I, Coindre JM. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol. 2005;29:1340–1347. doi: 10.1097/01.pas.0000170343.09562.39. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials