Rapid screening of gene function by systemic delivery of morpholino oligonucleotides to live mouse embryos
- PMID: 25629157
- PMCID: PMC4309589
- DOI: 10.1371/journal.pone.0114932
Rapid screening of gene function by systemic delivery of morpholino oligonucleotides to live mouse embryos
Abstract
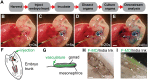
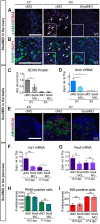
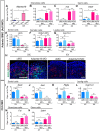
Traditional gene targeting methods in mice are complex and time consuming, especially when conditional deletion methods are required. Here, we describe a novel technique for assessing gene function by injection of modified antisense morpholino oligonucleotides (MOs) into the heart of mid-gestation mouse embryos. After allowing MOs to circulate through the embryonic vasculature, target tissues were explanted, cultured and analysed for expression of key markers. We established proof-of-principle by partially phenocopying known gene knockout phenotypes in the fetal gonads (Stra8, Sox9) and pancreas (Sox9). We also generated a novel double knockdown of Gli1 and Gli2, revealing defects in Leydig cell differentiation in the fetal testis. Finally, we gained insight into the roles of Adamts19 and Ctrb1, genes of unknown function in sex determination and gonadal development. These studies reveal the utility of this method as a means of first-pass analysis of gene function during organogenesis before committing to detailed genetic analysis.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

