Sustainable hydrogen photoproduction by phosphorus-deprived marine green microalgae Chlorella sp
- PMID: 25629229
- PMCID: PMC4346860
- DOI: 10.3390/ijms16022705
Sustainable hydrogen photoproduction by phosphorus-deprived marine green microalgae Chlorella sp
Abstract
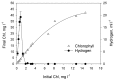
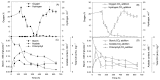
Previously it has been shown that green microalga Chlamydomonas reinhardtii is capable of prolonged H2 photoproduction when deprived of sulfur. In addition to sulfur deprivation (-S), sustained H2 photoproduction in C. reinhardtii cultures can be achieved under phosphorus-deprived (-P) conditions. Similar to sulfur deprivation, phosphorus deprivation limits O2 evolving activity in algal cells and causes other metabolic changes that are favorable for H2 photoproduction. Although significant advances in H2 photoproduction have recently been realized in fresh water microalgae, relatively few studies have focused on H2 production in marine green microalgae. In the present study phosphorus deprivation was applied for hydrogen production in marine green microalgae Chlorella sp., where sulfur deprivation is impossible due to a high concentration of sulfates in the sea water. Since resources of fresh water on earth are limited, the possibility of hydrogen production in seawater is more attractive. In order to achieve H2 photoproduction in P-deprived marine green microalgae Chlorella sp., the dilution approach was applied. Cultures diluted to about 0.5-1.8 mg Chl·L-1 in the beginning of P-deprivation were able to establish anaerobiosis, after the initial growth period, where cells utilize intracellular phosphorus, with subsequent transition to H2 photoproduction stage. It appears that marine microalgae during P-deprivation passed the same stages of adaptation as fresh water microalgae. The presence of inorganic carbon was essential for starch accumulation and subsequent hydrogen production by microalgae. The H2 accumulation was up to 40 mL H2 gas per 1iter of the culture, which is comparable to that obtained in P-deprived C. reinhardtii culture.
Figures


Similar articles
-
Hydrogen production by photoautotrophic sulfur-deprived Chlamydomonas reinhardtii pre-grown and incubated under high light.Biotechnol Bioeng. 2009 Mar 1;102(4):1055-61. doi: 10.1002/bit.22148. Biotechnol Bioeng. 2009. PMID: 18985615
-
Autotrophic hydrogen photoproduction by operation of carbon-concentrating mechanism in Chlamydomonas reinhardtii under sulfur deprivation condition.J Biotechnol. 2016 Mar 10;221:55-61. doi: 10.1016/j.jbiotec.2016.01.023. Epub 2016 Jan 23. J Biotechnol. 2016. PMID: 26812657
-
Autotrophic and mixotrophic hydrogen photoproduction in sulfur-deprived chlamydomonas cells.Appl Environ Microbiol. 2005 Oct;71(10):6199-205. doi: 10.1128/AEM.71.10.6199-6205.2005. Appl Environ Microbiol. 2005. PMID: 16204539 Free PMC article.
-
Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae).Planta. 2007 Oct;226(5):1075-86. doi: 10.1007/s00425-007-0609-9. Epub 2007 Aug 25. Planta. 2007. PMID: 17721788 Review.
-
Advances in the biotechnology of hydrogen production with the microalga Chlamydomonas reinhardtii.Crit Rev Biotechnol. 2015;35(4):485-96. doi: 10.3109/07388551.2014.900734. Epub 2014 Apr 22. Crit Rev Biotechnol. 2015. PMID: 24754449 Review.
Cited by
-
Multiple regulatory mechanisms in the chloroplast of green algae: relation to hydrogen production.Photosynth Res. 2015 Sep;125(3):357-81. doi: 10.1007/s11120-015-0157-2. Epub 2015 May 19. Photosynth Res. 2015. PMID: 25986411
-
Relevance of nutrient media composition for hydrogen production in Chlamydomonas.Photosynth Res. 2015 Sep;125(3):395-406. doi: 10.1007/s11120-015-0152-7. Epub 2015 May 8. Photosynth Res. 2015. PMID: 25952745 Review.
-
Outlook on Synthetic Biology-Driven Hydrogen Production: Lessons from Algal Photosynthesis Applied to Cyanobacteria.Energy Fuels. 2025 Mar 11;39(11):4987-5006. doi: 10.1021/acs.energyfuels.4c04772. eCollection 2025 Mar 20. Energy Fuels. 2025. PMID: 40134520 Free PMC article. Review.
-
Microalgal hydrogen production: prospects of an essential technology for a clean and sustainable energy economy.Photosynth Res. 2017 Sep;133(1-3):49-62. doi: 10.1007/s11120-017-0350-6. Epub 2017 Feb 26. Photosynth Res. 2017. PMID: 28239761 Free PMC article. Review.
-
Algae-Bacteria Consortia as a Strategy to Enhance H2 Production.Cells. 2020 May 29;9(6):1353. doi: 10.3390/cells9061353. Cells. 2020. PMID: 32486026 Free PMC article. Review.
References
-
- Tsygankov A. Hydrogen production: light driven processes—Green algae. In: Hallenbeck P.C., editor. Microbial Technologies in Advanced Biofuels Production. Springer; New York, NY, USA/Dordrecht, The Netherlands/Heidelberg, Germany/London, UK: 2012. pp. 29–52.
-
- Allahverdiyeva Y., Kosourov S. Recent Developments on Cyanobacteria and Green Algae for Biohydrogen Photoproduction and Its Importance in CO2 Reduction. In: Gupta V.K., Kubicek C.P., Saddler J., Xu F.F., Tuohy M.G., editors. Bioenergy Research: Advances and Applications. Elsevier; New York, NY, USA: 2014. pp. 389–406.
-
- Kosourov S., Batyrova K., Petushkova E., Tsygankov A., Ghirardi M.L., Seibert M. Maximizing the hydrogen photoproduction yields in Chlamydomonas. reinhardtii cultures: The effect of the H2 partial pressure. Int. J. Hydrog. Energy. 2012;37:8850–8858.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

