Human iPS cell-derived insulin producing cells form vascularized organoids under the kidney capsules of diabetic mice
- PMID: 25629318
- PMCID: PMC4309616
- DOI: 10.1371/journal.pone.0116582
Human iPS cell-derived insulin producing cells form vascularized organoids under the kidney capsules of diabetic mice
Abstract
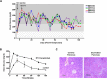
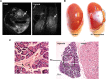
Type 1 diabetes (T1D) is caused by autoimmune disease that leads to the destruction of pancreatic β-cells. Transplantation of cadaveric pancreatic organs or pancreatic islets can restore normal physiology. However, there is a chronic shortage of cadaveric organs, limiting the treatment of the majority of patients on the pancreas transplantation waiting list. Here, we hypothesized that human iPS cells can be directly differentiated into insulin producing cells (IPCs) capable of secreting insulin. Using a series of pancreatic growth factors, we successfully generated iPS cells derived IPCs. Furthermore, to investigate the capability of these cells to secrete insulin in vivo, the differentiated cells were transplanted under the kidney capsules of diabetic immunodeficient mice. Serum glucose levels gradually declined to either normal or near normal levels over 150 days, suggesting that the IPCs were secreting insulin. In addition, using MRI, a 3D organoid appeared as a white patch on the transplanted kidneys but not on the control kidneys. These organoids showed neo-vascularization and stained positive for insulin and glucagon. All together, these data show that a pancreatic organ can be created in vivo providing evidence that iPS cells might be a novel option for the treatment of T1D.
Conflict of interest statement
Figures




References
-
- Ben Nasr M, D’Addio F, Usuelli V, Tezza S, Abdi R, et al. (2014) The rise, fall, and resurgence of immunotherapy in type 1 diabetes. Pharmacol Res. - PubMed
-
- Dao LT, Park EY, Lim SM, Choi YS, Jung HS, et al. (2014) Transplantation of Insulin-Producing Cells Differentiated from Human Periosteum-Derived Progenitor Cells Ameliorate Hyperglycemia in Diabetic Mice. Transplantation. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

