Wogonin induces eosinophil apoptosis and attenuates allergic airway inflammation
- PMID: 25629436
- PMCID: PMC4384778
- DOI: 10.1164/rccm.201408-1565OC
Wogonin induces eosinophil apoptosis and attenuates allergic airway inflammation
Abstract
Rationale: Eosinophils are key effector cells in allergic diseases, including allergic rhinitis, eczema, and asthma. Their tissue presence is regulated by both recruitment and increased longevity at inflamed sites.
Objectives: To investigate the ability of the flavone wogonin to induce eosinophil apoptosis in vitro and attenuate eosinophil-dominant allergic inflammation in vivo in mice.
Methods: Human and mouse eosinophil apoptosis in response to wogonin was investigated by cellular morphology, flow cytometry, mitochondrial membrane permeability, and pharmacological caspase inhibition. Allergic lung inflammation was modeled in mice sensitized and challenged with ovalbumin. Bronchoalveolar lavage (BAL) and lung tissue were examined for inflammation, mucus production, and inflammatory mediator production. Airway hyperresponsiveness to aerosolized methacholine was measured.
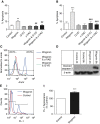
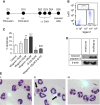
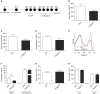
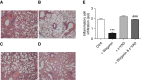
Measurements and main results: Wogonin induced time- and concentration-dependent human and mouse eosinophil apoptosis in vitro. Wogonin-induced eosinophil apoptosis occurred with activation of caspase-3 and was inhibited by pharmacological caspase inhibition. Wogonin administration attenuated allergic airway inflammation in vivo with reductions in BAL and interstitial eosinophil numbers, increased eosinophil apoptosis, reduced airway mucus production, and attenuated airway hyperresponsiveness. This wogonin-induced reduction in allergic airway inflammation was prevented by concurrent caspase inhibition in vivo.
Conclusions: Wogonin induces eosinophil apoptosis and attenuates allergic airway inflammation, suggesting that it has therapeutic potential for the treatment of allergic inflammation in humans.
Keywords: airway resistance; antiinflammatory agents; hypersensitivity; inflammation resolution; mucus.
Figures



Comment in
-
Drug-induced death of eosinophils. Promises and pitfalls.Am J Respir Crit Care Med. 2015 Mar 15;191(6):605-6. doi: 10.1164/rccm.201501-0156ED. Am J Respir Crit Care Med. 2015. PMID: 25767918 No abstract available.
References
-
- Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. - PubMed
-
- Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

