Convergent evolution of AUA decoding in bacteria and archaea
- PMID: 25629511
- PMCID: PMC4615378
- DOI: 10.4161/15476286.2014.992281
Convergent evolution of AUA decoding in bacteria and archaea
Abstract
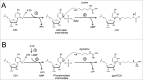
Deciphering AUA codons is a difficult task for organisms, because AUA and AUG specify isoleucine (Ile) and methionine (Met), separately. Each of the other purine-ending sense co-don sets (NNR) specifies a single amino acid in the universal genetic code. In bacteria and archaea, the cytidine derivatives, 2-lysylcytidine (L or lysidine) and 2-agmatinylcytidine (agm(2)C or agmatidine), respectively, are found at the first letter of the anticodon of tRNA(Ile) responsible for AUA codons. These modifications prevent base pairing with G of the third letter of AUG codon, and enable tRNA(Ile) to decipher AUA codon specifically. In addition, these modifications confer a charging ability of tRNA(Ile) with Ile. Despite their similar chemical structures, L and agm(2)C are synthesized by distinctive mechanisms and catalyzed by different classes of enzymes, implying that the analogous decoding systems for AUA codons were established by convergent evolution after the phylogenic split between bacteria and archaea-eukaryotes lineages following divergence from the last universal common ancestor (LUCA).
Figures





Similar articles
-
Mechanisms of the tRNA wobble cytidine modification essential for AUA codon decoding in prokaryotes.Biosci Biotechnol Biochem. 2015;79(3):347-53. doi: 10.1080/09168451.2014.975185. Epub 2014 Oct 28. Biosci Biotechnol Biochem. 2015. PMID: 25348586 Review.
-
Structural insights into the decoding capability of isoleucine tRNAs with lysidine and agmatidine.Nat Struct Mol Biol. 2024 May;31(5):817-825. doi: 10.1038/s41594-024-01238-1. Epub 2024 Mar 27. Nat Struct Mol Biol. 2024. PMID: 38538915
-
Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea.Nat Chem Biol. 2010 Apr;6(4):277-82. doi: 10.1038/nchembio.323. Epub 2010 Feb 7. Nat Chem Biol. 2010. PMID: 20139989
-
A tRNA modification with aminovaleramide facilitates AUA decoding in protein synthesis.Nat Chem Biol. 2025 Apr;21(4):522-531. doi: 10.1038/s41589-024-01726-x. Epub 2024 Sep 19. Nat Chem Biol. 2025. PMID: 39300229 Free PMC article.
-
Enzymatic conversion of cytidine to lysidine in anticodon of bacterial isoleucyl-tRNA--an alternative way of RNA editing.Trends Biochem Sci. 2004 Apr;29(4):165-8. doi: 10.1016/j.tibs.2004.02.009. Trends Biochem Sci. 2004. PMID: 15124629 Review. No abstract available.
Cited by
-
Regulatory Factors for tRNA Modifications in Extreme- Thermophilic Bacterium Thermus thermophilus.Front Genet. 2019 Mar 8;10:204. doi: 10.3389/fgene.2019.00204. eCollection 2019. Front Genet. 2019. PMID: 30906314 Free PMC article. Review.
-
Polyamine function in archaea and bacteria.J Biol Chem. 2018 Nov 30;293(48):18693-18701. doi: 10.1074/jbc.TM118.005670. Epub 2018 Sep 25. J Biol Chem. 2018. PMID: 30254075 Free PMC article. Review.
-
"Superwobbling" and tRNA-34 Wobble and tRNA-37 Anticodon Loop Modifications in Evolution and Devolution of the Genetic Code.Life (Basel). 2022 Feb 8;12(2):252. doi: 10.3390/life12020252. Life (Basel). 2022. PMID: 35207539 Free PMC article. Review.
-
The mechanism of discriminative aminoacylation by isoleucyl-tRNA synthetase based on wobble nucleotide recognition.Nat Commun. 2024 Dec 30;15(1):10817. doi: 10.1038/s41467-024-55183-0. Nat Commun. 2024. PMID: 39738040 Free PMC article.
-
Matching tRNA modifications in humans to their known and predicted enzymes.Nucleic Acids Res. 2019 Mar 18;47(5):2143-2159. doi: 10.1093/nar/gkz011. Nucleic Acids Res. 2019. PMID: 30698754 Free PMC article.
References
-
- Ogle JM, Murphy FV, Tarry MJ, Ramakrishnan V. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell 2002; 111:721-32; PMID:12464183; http://dx.doi.org/10.1016/S0092-8674(02)01086-3 - DOI - PubMed
-
- Ogle JM, Brodersen DE, Clemons WM, Jr, Tarry MJ, Carter AP, Ramakrishnan V. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 2001; 292:897-902; PMID:11340196; http://dx.doi.org/10.1126/science.1060612 - DOI - PubMed
-
- Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FV, 4th, Weir JR, Ramakrishnan V. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 2009; 326:688-94; PMID:19833920; http://dx.doi.org/10.1126/science.1179700 - DOI - PMC - PubMed
-
- Voorhees RM, Schmeing TM, Kelley AC, Ramakrishnan V. The mechanism for activation of GTP hydrolysis on the ribosome. Science 2010; 330:835-8; PMID:21051640; http://dx.doi.org/10.1126/science.1194460 - DOI - PMC - PubMed
-
- Crick FH. Codon–anticodon pairing: the wobble hypothesis. J Mol Biol 1966; 19:548-55; PMID:5969078; http://dx.doi.org/10.1016/S0022-2836(66)80022-0 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
