Detection of benzo[a]pyrene-guanine adducts in single-stranded DNA using the α-hemolysin nanopore
- PMID: 25629967
- PMCID: PMC5266612
- DOI: 10.1088/0957-4484/26/7/074002
Detection of benzo[a]pyrene-guanine adducts in single-stranded DNA using the α-hemolysin nanopore
Abstract
The carcinogenic precursor benzo[a]pyrene (BP), a polycyclic aromatic hydrocarbon, is released into the environment through the incomplete combustion of hydrocarbons. Metabolism of BP in the human body yields a potent alkylating agent (benzo[a]pyrene diol epoxide, BPDE) that reacts with guanine (G) in DNA to form an adduct implicated in cancer initiation. We report that the α-hemolysin (αHL) nanopore platform can be used to detect a BPDE adduct to G in synthetic oligodeoxynucleotides. Translocation of a 41-mer poly-2'-deoxycytidine strand with a centrally located BPDE adduct to G through αHL in 1 M KCl produces a unique multi-level current signature allowing the adduct to be detected. This readily distinguishable current modulation was observed when the BPDE-adducted DNA strand translocated from either the 5' or 3' directions. This study suggests that BPDE adducts and other large aromatic biomarkers can be detected with αHL, presenting opportunities for the monitoring, quantification, and sequencing of mutagenic compounds from cellular DNA samples.
Figures
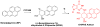



References
-
- Toyooka T, Ibuki Y. DNA damage induced by coexposure to PAHs and light. Environ Toxicol Pharmacol. 2007;23:256–263. - PubMed
-
- Baird WM, Hooven LA, Mahadevan B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ Mol Mutagen. 2005;45:106–114. - PubMed
-
- Phillips DH. Fifty years of benzo(a)pyrene. Nature. 1983;303:468–472. - PubMed
-
- Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer causes control. 1997;8:444–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
