Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease
- PMID: 25630029
- PMCID: PMC4366346
- DOI: 10.1097/j.pain.0000000000000104
Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease
Abstract
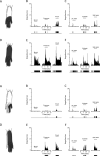
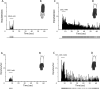
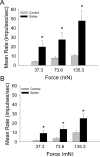
Chronic pain is a major characteristic feature of sickle cell disease (SCD). The refractory nature of pain and the development of chronic pain syndromes in many patients with SCD suggest that central neural mechanisms contribute to pain in this disease. We used HbSS-BERK sickle mice, which show chronic features of pain similar to those observed in SCD, and determined whether sensitization of nociceptive neurons in the spinal cord contributes to pain and hyperalgesia in SCD. Electrophysiological recordings of action potential activity were obtained from single identified dorsal horn neurons of the spinal cord in anesthetized mice. Compared with control HbAA-BERK mice, nociceptive dorsal horn neurons in sickle mice exhibited enhanced excitability as evidenced by enlarged receptive fields, increased rate of spontaneous activity, lower mechanical thresholds, enhanced responses to mechanical stimuli, and prolonged afterdischarges following mechanical stimulation. These changes were accompanied by increased phosphorylation of mitogen-activated protein kinases (MAPKs) in the spinal cord that are known to contribute to neuronal hyperexcitability, including c-Jun N-terminal kinase (JNK), p44/p42 extracellular signaling-regulated kinase (ERK), and p38. These findings demonstrate that central sensitization contributes to pain in SCD.
Figures

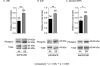
References
-
- Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120:3647–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

