Effect of tyrosine autophosphorylation on catalytic activity and subcellular localisation of homeodomain-interacting protein kinases (HIPK)
- PMID: 25630557
- PMCID: PMC4353451
- DOI: 10.1186/s12964-014-0082-6
Effect of tyrosine autophosphorylation on catalytic activity and subcellular localisation of homeodomain-interacting protein kinases (HIPK)
Abstract
Background: Homeodomain interacting protein kinases (HIPKs) function as modulators of cellular stress responses and regulate cell differentiation, proliferation and apoptosis. The HIPK family includes HIPK1, HIPK2 and HIPK3, which share a similar domain structure, and the more distantly related HIPK4. Although HIPKs phosphorylate their substrates on serine or threonine residues, it was recently reported that HIPK2 depends on the autophosphorylation of a conserved tyrosine in the activation loop to acquire full catalytic activity and correct subcellular localization. In this study we addressed the question whether tyrosine autophosphorylation in the activation loop has a similar function in the other members of the HIPK family.
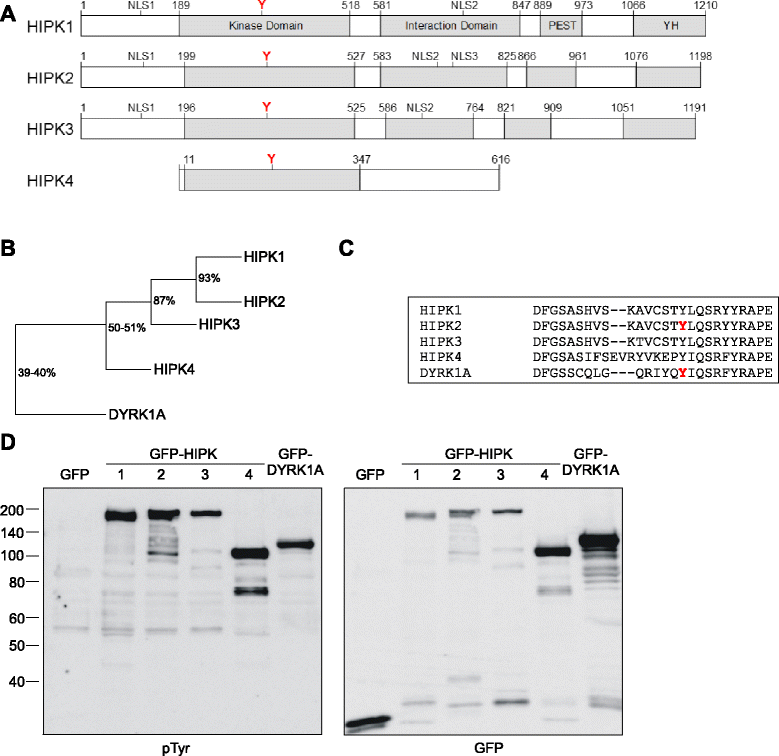
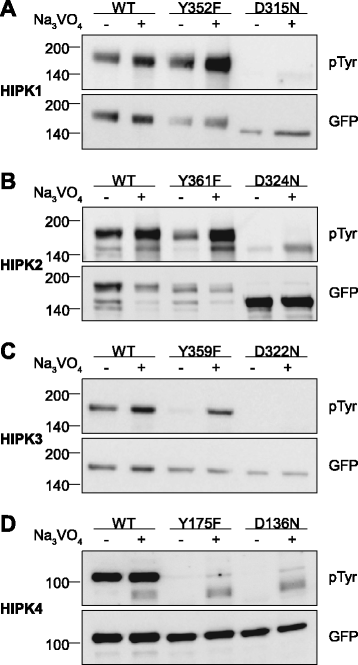
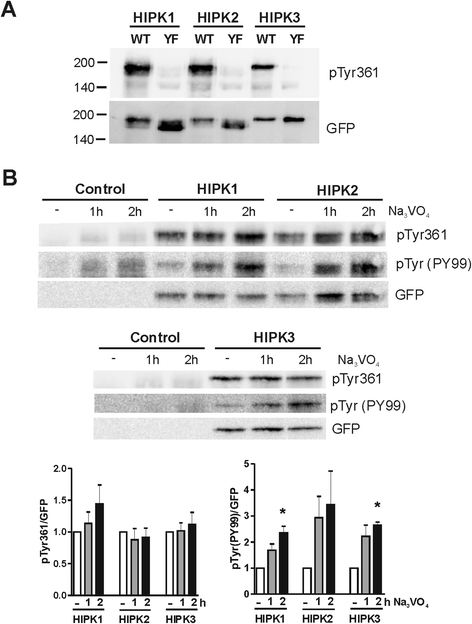
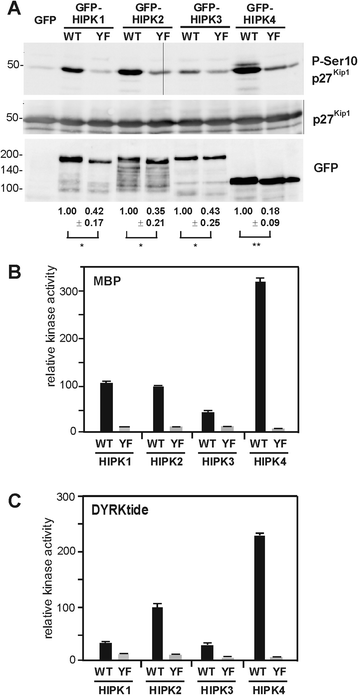
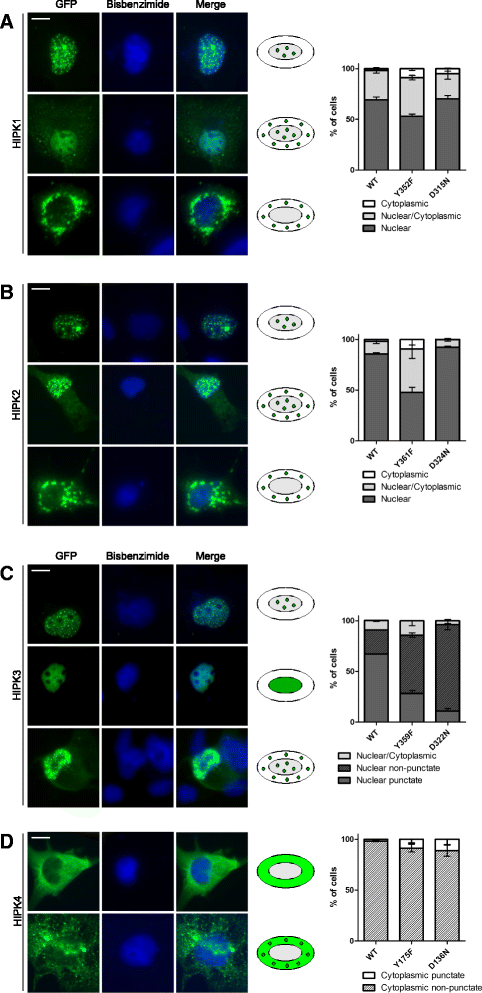
Results: All HIPKs contained phosphotyrosine when expressed in HeLa cells. Catalytically inactive point mutants were not tyrosine-phosphorylated, indicating that HIPKs are dual-specificity protein kinases that autophosphorylate on tyrosine residues. HIPK point mutants lacking the conserved tyrosine residue in the activation loop showed reduced catalytic activity towards peptide and protein substrates. Analysis of these mutants revealed that HIPK1, HIPK2 and HIPK3 but not HIPK4 are capable of autophosphorylating on other tyrosines. Inhibition of tyrosine phosphatase activity by treatment with vanadate enhanced global phosphotyrosine content of HIPK1, HIPK2 and HIPK3 but did not affect tyrosine phosphorylation in the activation loop. Mutation of the activation-loop tyrosines resulted in a redistribution of HIPK1 and HIPK2 from a speckle-like subnuclear compartment to the cytoplasm, whereas catalytically inactive point mutants showed the same pattern of cellular distribution as the wild type proteins. In contrast, mutation of the activating tyrosine did not increase the low percentage of cells with extranuclear HIPK3. HIPK4 was excluded from the nucleus with no difference between the wild type kinase and the point mutants.
Conclusions: These results show that HIPKs share the mechanism of activation by tyrosine autophosphorylation with the closely related DYRK family (dual-specificity tyrosine phosphorylation regulated kinase). However, members of the HIPK family differ regarding the subcellular localization and its dependence on tyrosine autophosphorylation.
Figures
Similar articles
-
Mechanism of dual specificity kinase activity of DYRK1A.FEBS J. 2013 Sep;280(18):4495-511. doi: 10.1111/febs.12411. Epub 2013 Jul 22. FEBS J. 2013. PMID: 23809146
-
Characterization of Human Homeodomain-interacting Protein Kinase 4 (HIPK4) as a Unique Member of the HIPK Family.Mol Cell Pharmacol. 2010;2(2):61-68. Mol Cell Pharmacol. 2010. PMID: 20508833 Free PMC article.
-
HIPK2 kinase activity depends on cis-autophosphorylation of its activation loop.J Mol Cell Biol. 2013 Feb;5(1):27-38. doi: 10.1093/jmcb/mjs053. Epub 2012 Sep 20. J Mol Cell Biol. 2013. PMID: 23000554
-
Update on the Regulation of HIPK1, HIPK2 and HIPK3 Protein Kinases by microRNAs.Microrna. 2018;7(3):178-186. doi: 10.2174/2211536607666180525102330. Microrna. 2018. PMID: 29793420 Review.
-
Integration of stress signals by homeodomain interacting protein kinases.Biol Chem. 2014 Apr;395(4):375-86. doi: 10.1515/hsz-2013-0264. Biol Chem. 2014. PMID: 24225127 Review.
Cited by
-
HIPK family kinases bind and regulate the function of the CCR4-NOT complex.Mol Biol Cell. 2016 Jun 15;27(12):1969-80. doi: 10.1091/mbc.E15-09-0629. Epub 2016 Apr 27. Mol Biol Cell. 2016. PMID: 27122605 Free PMC article.
-
miR-146b-5p activation of hepatic stellate cells contributes to the progression of fibrosis by directly targeting HIPK1.Exp Ther Med. 2022 Jun 27;24(2):537. doi: 10.3892/etm.2022.11474. eCollection 2022 Aug. Exp Ther Med. 2022. PMID: 35837064 Free PMC article.
-
Arsenic-Induced Activation of the Homeodomain-Interacting Protein Kinase 2 (HIPK2) to cAMP-Response Element Binding Protein (CREB) Axis.J Mol Biol. 2017 Jan 6;429(1):64-78. doi: 10.1016/j.jmb.2016.11.015. Epub 2016 Nov 21. J Mol Biol. 2017. PMID: 27884605 Free PMC article.
-
Expression of human HIPKs in Drosophila demonstrates their shared and unique functions in a developmental model.G3 (Bethesda). 2021 Dec 8;11(12):jkab350. doi: 10.1093/g3journal/jkab350. G3 (Bethesda). 2021. PMID: 34849772 Free PMC article.
-
The adaptor protein DCAF7 mediates the interaction of the adenovirus E1A oncoprotein with the protein kinases DYRK1A and HIPK2.Sci Rep. 2016 Jun 16;6:28241. doi: 10.1038/srep28241. Sci Rep. 2016. PMID: 27307198 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

