Oncolytic parvoviruses: from basic virology to clinical applications
- PMID: 25630937
- PMCID: PMC4323056
- DOI: 10.1186/s12985-014-0223-y
Oncolytic parvoviruses: from basic virology to clinical applications
Abstract
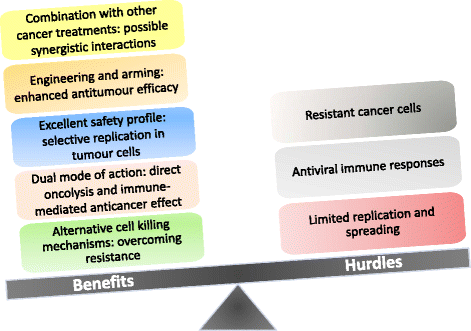
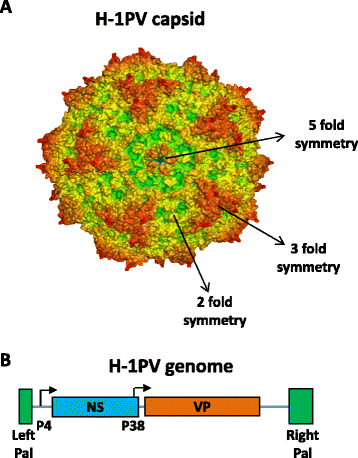
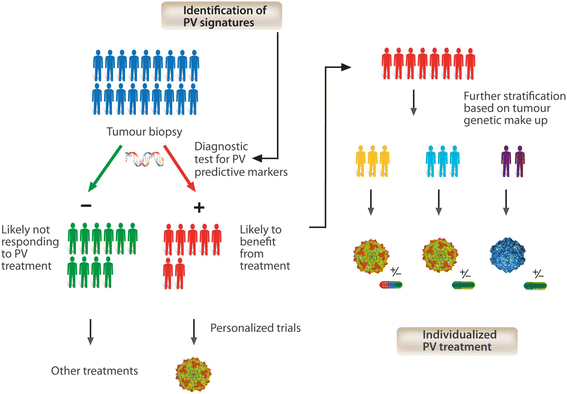
Accumulated evidence gathered over recent decades demonstrated that some members of the Parvoviridae family, in particular the rodent protoparvoviruses H-1PV, the minute virus of mice and LuIII have natural anticancer activity while being nonpathogenic to humans. These studies have laid the foundations for the launch of a first phase I/IIa clinical trial, in which the rat H-1 parvovirus is presently undergoing evaluation for its safety and first signs of efficacy in patients with glioblastoma multiforme. After a brief overview of the biology of parvoviruses, this review focuses on the studies which unraveled the antineoplastic properties of these agents and supported their clinical use as anticancer therapeutics. Furthermore, the development of novel parvovirus-based anticancer strategies with enhanced specificity and efficacy is discussed, in particular the development of second and third generation vectors and the combination of parvoviruses with other anticancer agents. Lastly, we address the key challenges that remain towards a more rational and efficient use of oncolytic parvoviruses in clinical settings, and discuss how a better understanding of the virus life-cycle and of the cellular factors involved in virus infection, replication and cytotoxicity may promote the further development of parvovirus-based anticancer therapies, open new prospects for treatment and hopefully improve clinical outcome.
Figures
References
-
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–659. - PubMed
-
- Kyula JN, Roulstone V, Karapanagiotou EM, Melcher AA, Harrington KJ. Oncolytic reovirus type 3 (Dearing) as a novel therapy in head and neck cancer. Expert Opin Biol Ther. 2012;12:1669–1678. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

