Clathrin mediates infectious hepatitis C virus particle egress
- PMID: 25631092
- PMCID: PMC4442374
- DOI: 10.1128/JVI.03620-14
Clathrin mediates infectious hepatitis C virus particle egress
Abstract
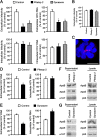
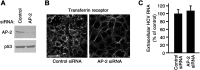
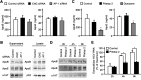
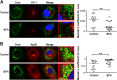
Although it is well established that hepatitis C virus (HCV) entry into hepatocytes depends on clathrin-mediated endocytosis, the possible roles of clathrin in other steps of the viral cycle remain unexplored. Thus, we studied whether cell culture-derived HCV (HCVcc) exocytosis was altered after clathrin interference. Knockdown of clathrin or the clathrin adaptor AP-1 in HCVcc-infected human hepatoma cell cultures impaired viral secretion without altering intracellular HCVcc levels or apolipoprotein B (apoB) and apoE exocytosis. Similar reductions in HCVcc secretion were observed after treatment with specific clathrin and dynamin inhibitors. Furthermore, detergent-free immunoprecipitation assays, neutralization experiments, and immunofluorescence analyses suggested that whereas apoE associated with infectious intracellular HCV precursors in endoplasmic reticulum (ER)-related structures, AP-1 participated in HCVcc egress in a post-ER compartment. Finally, we observed that clathrin and AP-1 knockdown altered the endosomal distribution of HCV core, reducing and increasing its colocalization with early endosome and lysosome markers, respectively. Our data support a model in which nascent HCV particles associate with apoE in the ER and exit cells following a clathrin-dependent transendosomal secretory route.
Importance: HCV entry into hepatocytes depends on clathrin-mediated endocytosis. Here we demonstrate for the first time that clathrin also participates in HCV exit from infected cells. Our data uncover important features of HCV egress, which may lead to the development of new therapeutic interventions. Interestingly, we show that secretion of the very-low-density lipoprotein (VLDL) components apoB and apoE is not impaired after clathrin interference. This is a significant finding, since, to date, it has been proposed that HCV and VLDL follow similar exocytic routes. Given that lipid metabolism recently emerged as a potential target for therapies against HCV infection, our data may help in the design of new strategies to interfere specifically with HCV exocytosis without perturbing cellular lipid homeostasis, with the aim of achieving more efficient, selective, and safe antivirals.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Hishiki T, Shimizu Y, Tobita R, Sugiyama K, Ogawa K, Funami K, Ohsaki Y, Fujimoto T, Takaku H, Wakita T, Baumert TF, Miyanari Y, Shimotohno K. 2010. Infectivity of hepatitis C virus is influenced by association with apolipoprotein E isoforms. J Virol 84:12048–12057. doi:10.1128/JVI.01063-10. - DOI - PMC - PubMed

