TREM2 regulates microglial cell activation in response to demyelination in vivo
- PMID: 25631124
- PMCID: PMC4667728
- DOI: 10.1007/s00401-015-1388-1
TREM2 regulates microglial cell activation in response to demyelination in vivo
Abstract
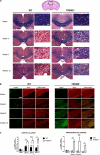
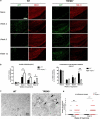
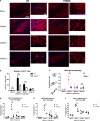
Microglia are phagocytic cells that survey the brain and perform neuroprotective functions in response to tissue damage, but their activating receptors are largely unknown. Triggering receptor expressed on myeloid cells 2 (TREM2) is a microglial immunoreceptor whose loss-of-function mutations in humans cause presenile dementia, while genetic variants are associated with increased risk of neurodegenerative diseases. In myeloid cells, TREM2 has been involved in the regulation of phagocytosis, cell proliferation and inflammatory responses in vitro. However, it is unknown how TREM2 contributes to microglia function in vivo. Here, we identify a critical role for TREM2 in the activation and function of microglia during cuprizone (CPZ)-induced demyelination. TREM2-deficient (TREM2(-/-)) mice had defective clearance of myelin debris and more axonal pathology, resulting in impaired clinical performances compared to wild-type (WT) mice. TREM2(-/-) microglia proliferated less in areas of demyelination and were less activated, displaying a more resting morphology and decreased expression of the activation markers MHC II and inducible nitric oxide synthase as compared to WT. Mechanistically, gene expression and ultrastructural analysis of microglia suggested a defect in myelin degradation and phagosome processing during CPZ intoxication in TREM2(-/-) microglia. These findings place TREM2 as a key regulator of microglia activation in vivo in response to tissue damage.
Figures

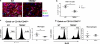

References
-
- Archambault AS, Sim J, McCandless EE, Klein RS, Russell JH. Region-specific regulation of inflammation and pathogenesis in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;181:122–132. doi:10.1016/j.jneuroim.2006.08.012. - PubMed
-
- Bayer TA. Proteinopathies, a core concept for understanding and ultimately treating degenerative disorders? Eur Neuropsychopharmacol. 2013 doi:10.1016/j.euroneuro.2013.03.007. - PubMed
-
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer's disease. Brain. 2009;132:1078–1092. doi:10.1093/brain/awn331. - PubMed
-
- Borroni B, Ferrari F, Galimberti D, Nacmias B, Barone C, Bagnoli S, Fenoglio C, Piaceri I, Archetti S, Bonvicini C, et al. Heterozygous TREM2 mutations in frontotemporal dementia. Neurobiol Aging. 2014;35(934):e937. doi:10.1016/j.neurobiolaging.2013.09.017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

