Yeast prions: structure, biology, and prion-handling systems
- PMID: 25631286
- PMCID: PMC4402965
- DOI: 10.1128/MMBR.00041-14
Yeast prions: structure, biology, and prion-handling systems
Abstract
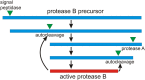
A prion is an infectious protein horizontally transmitting a disease or trait without a required nucleic acid. Yeast and fungal prions are nonchromosomal genes composed of protein, generally an altered form of a protein that catalyzes the same alteration of the protein. Yeast prions are thus transmitted both vertically (as genes composed of protein) and horizontally (as infectious proteins, or prions). Formation of amyloids (linear ordered β-sheet-rich protein aggregates with β-strands perpendicular to the long axis of the filament) underlies most yeast and fungal prions, and a single prion protein can have any of several distinct self-propagating amyloid forms with different biological properties (prion variants). Here we review the mechanism of faithful templating of protein conformation, the biological roles of these prions, and their interactions with cellular chaperones, the Btn2 and Cur1 aggregate-handling systems, and other cellular factors governing prion generation and propagation. Human amyloidoses include the PrP-based prion conditions and many other, more common amyloid-based diseases, several of which show prion-like features. Yeast prions increasingly are serving as models for the understanding and treatment of many mammalian amyloidoses. Patients with different clinical pictures of the same amyloidosis may be the equivalent of yeasts with different prion variants.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

